Int J Stem Cells.
2014 Nov;7(2):70-78. 10.15283/ijsc.2014.7.2.70.
Enhancement of Neural Stem Cells after Induction of Depression in Male Albino Rats (A histological & Immunohistochemical Study)
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Cairo University, Cairo, Egypt. gamalshaimaa@rocketmail.com
- KMID: 1974594
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.70
Abstract
- BACKGROUND AND OBJECTIVES
Depression is one of the most prevalent psychiatric disorders. Endogenous neural stem cells (NSCs) could replace damaged Hippocampal neurons in depression. This work was planned to evaluate Rhodiola rosea (Rr) extract possible role in stimulation of NSCs proliferation and in depression improvement.
METHODS AND RESULTS
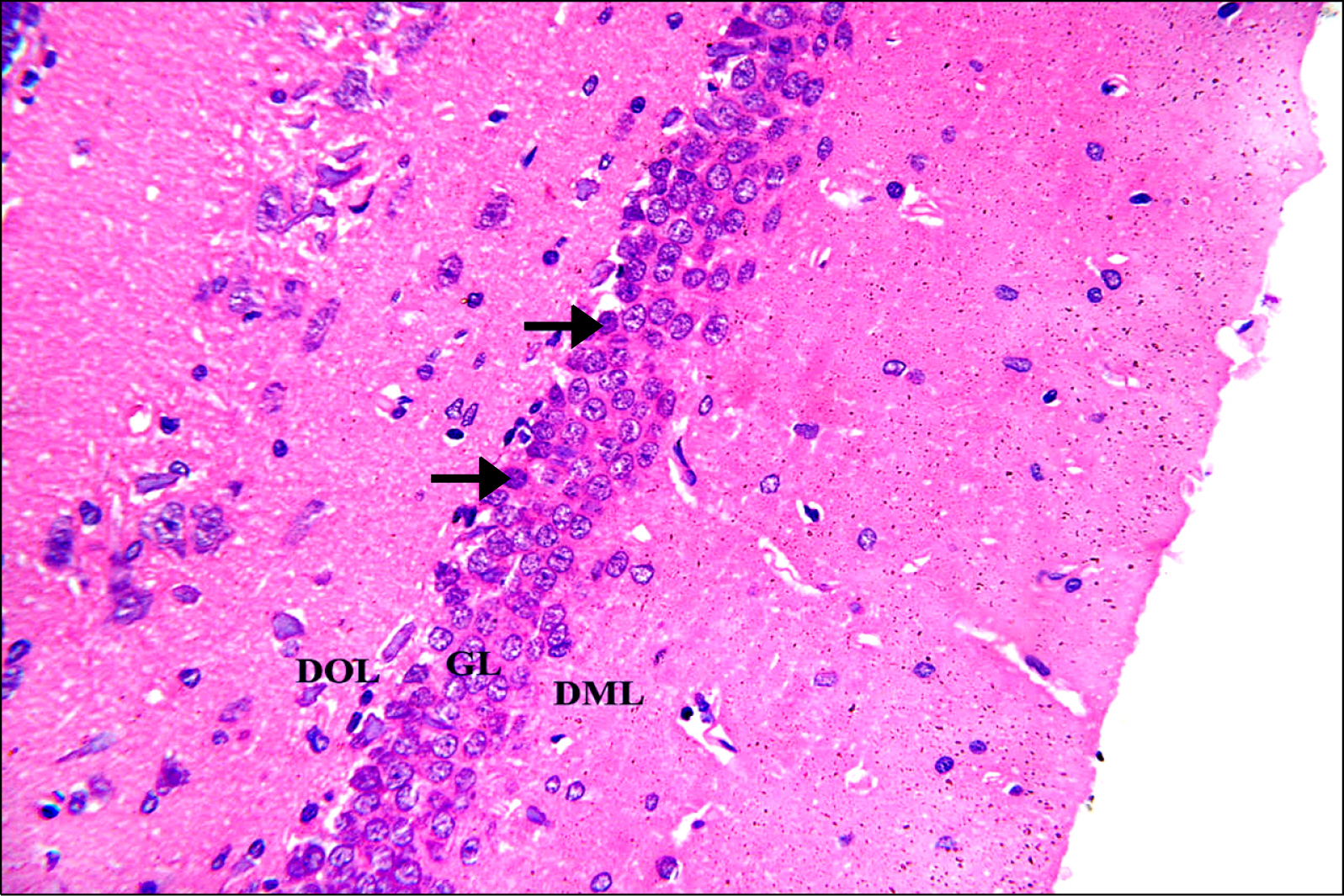
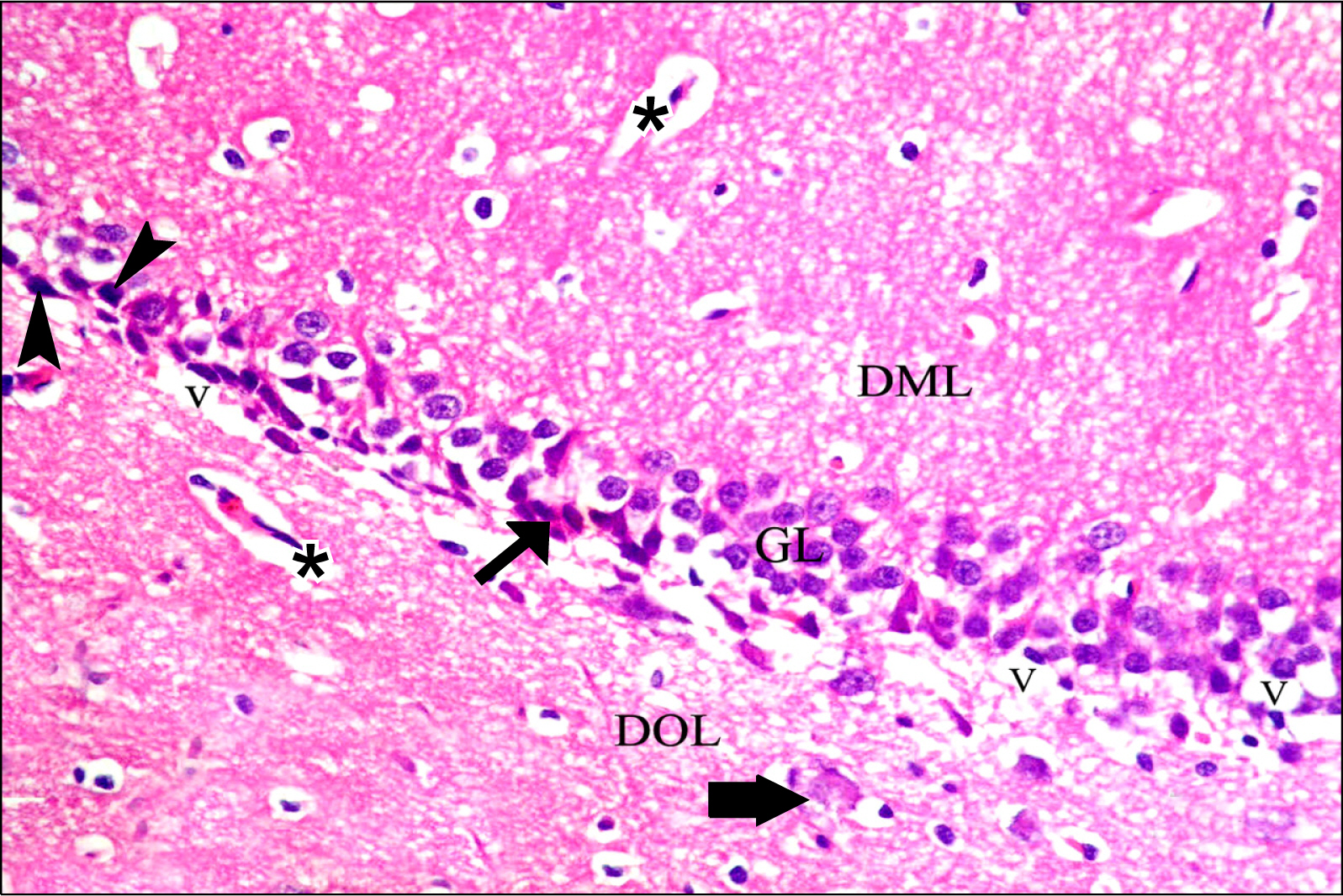
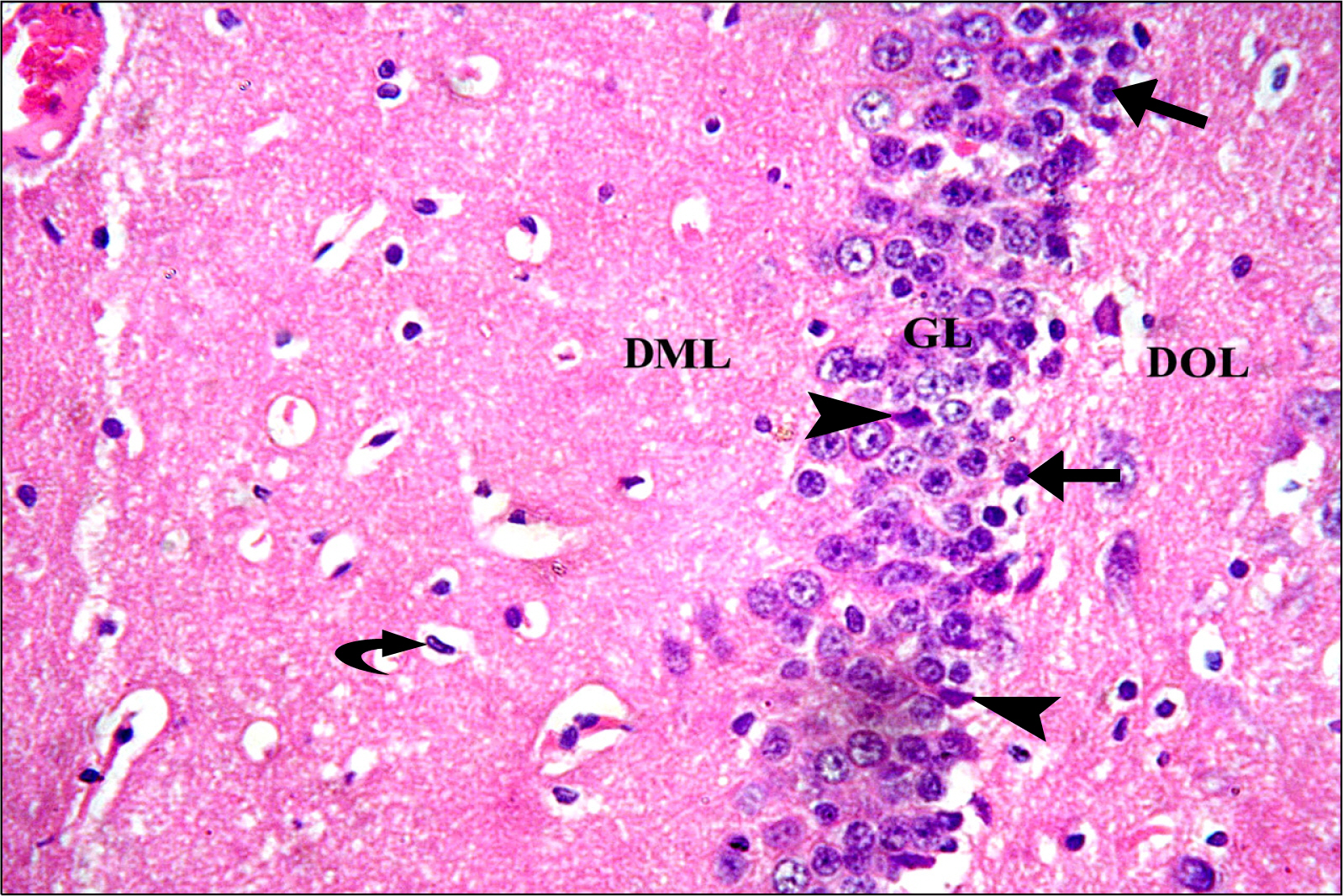
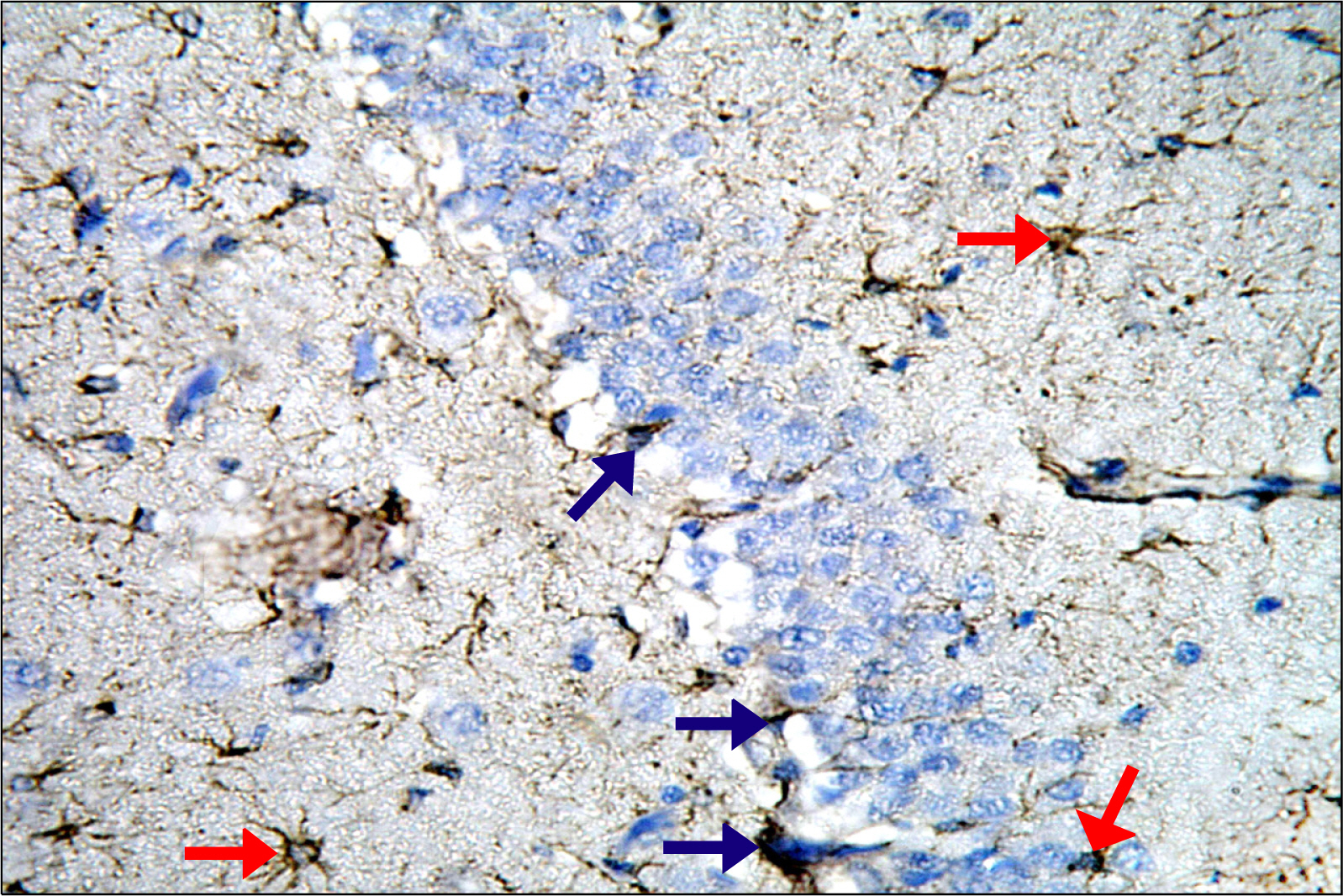
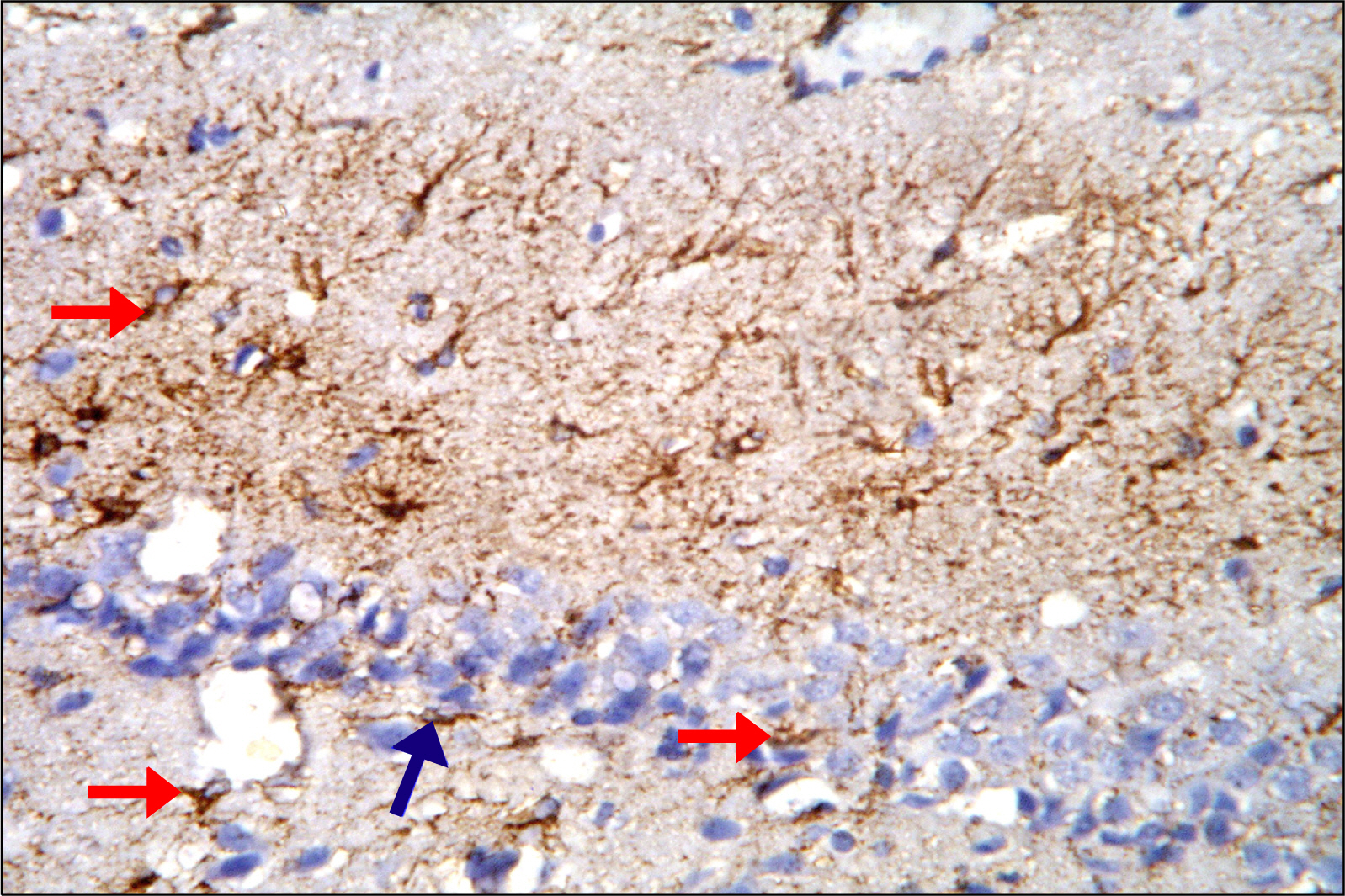
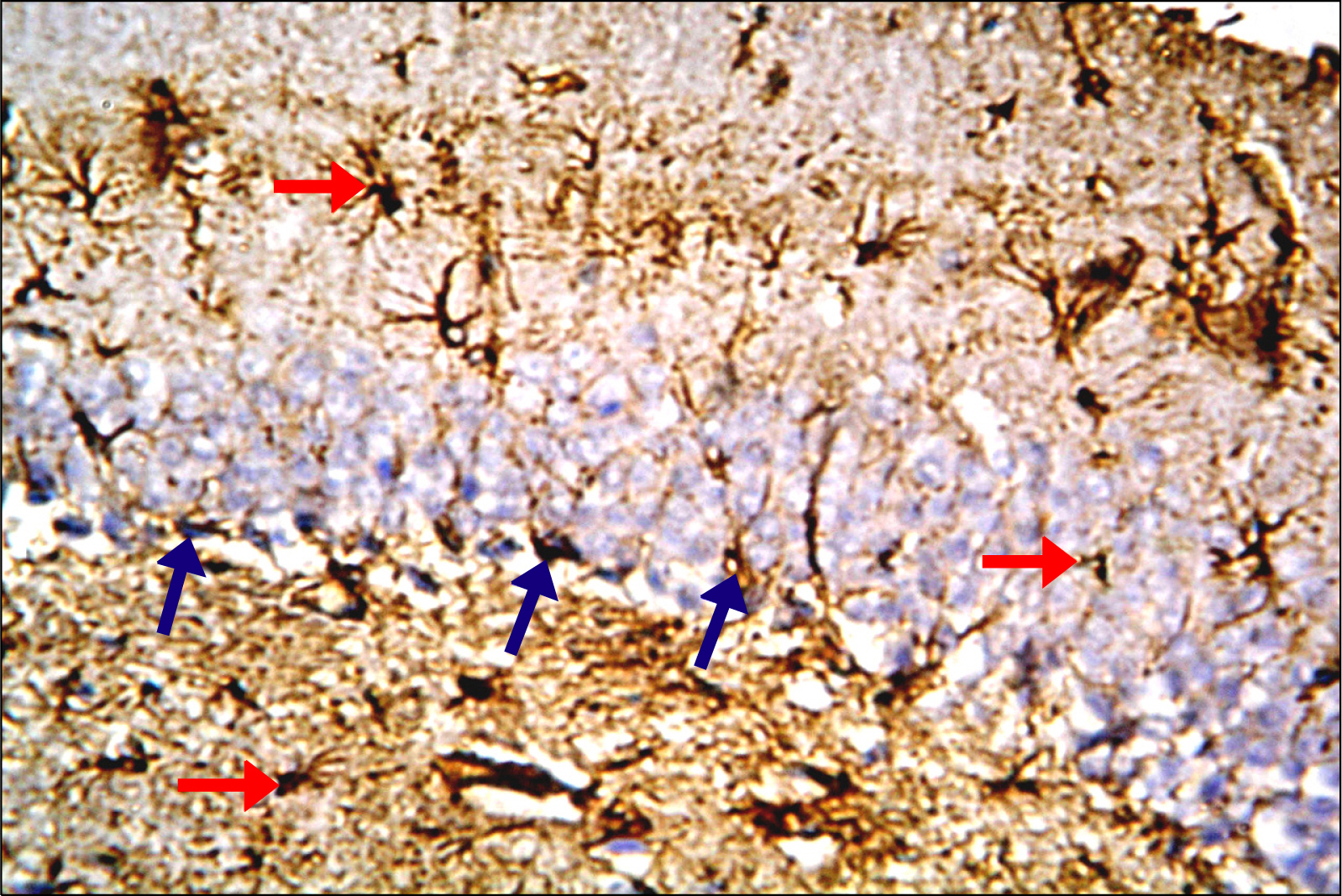
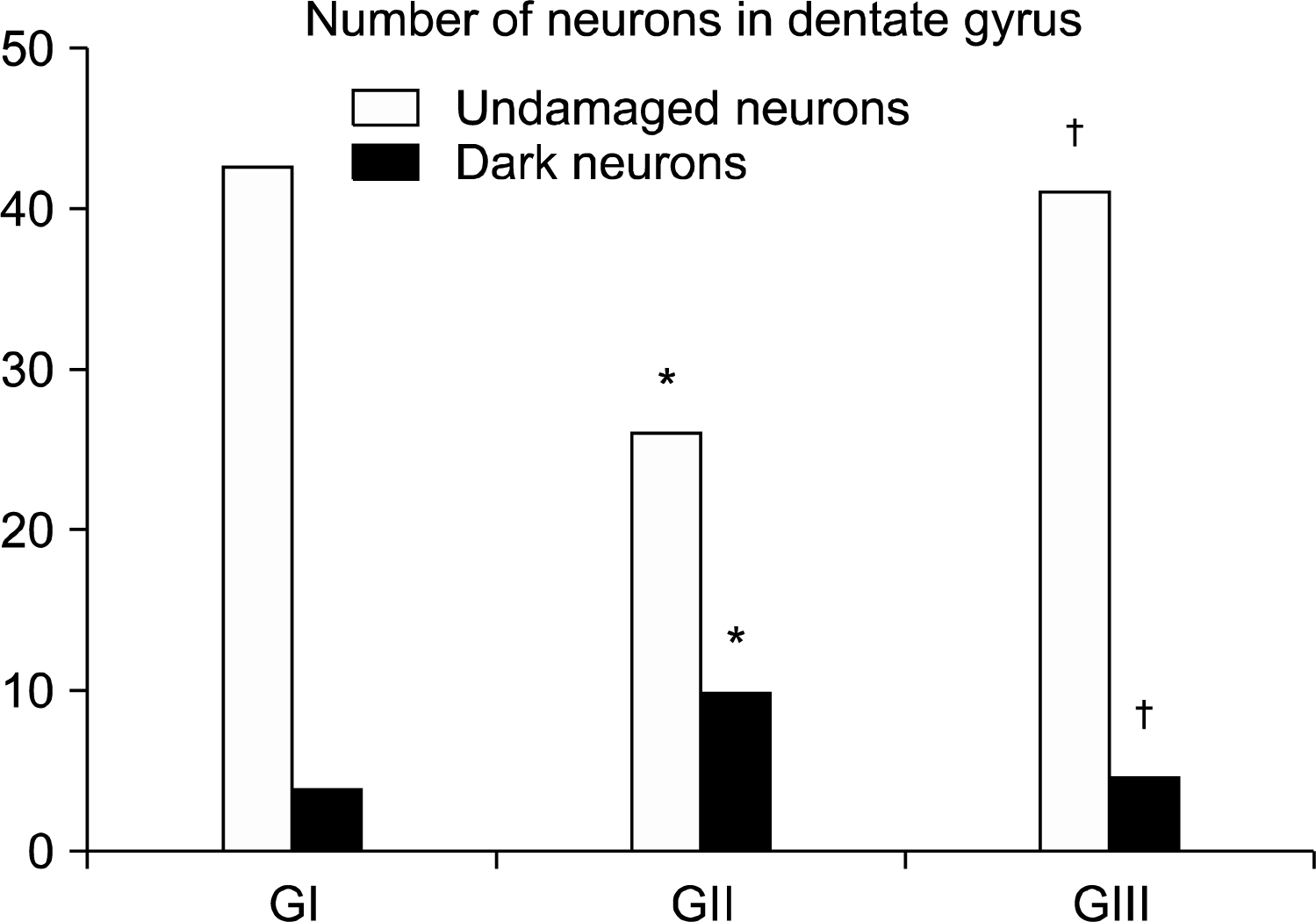
Thirty adult male albino rats were divided into three groups; control, untreated depressed model and Rr model. After depression induction by chronic mild stress, rats received Rr extract 1.5 g/kg/day for three weeks. The sucrose preference test (SP) was done before, after depression induction and 3 weeks after supplementation of Rr. The brain was removed and processed for H&E and immunohistochemical staining for caspase 3, glial fibrillary acid protein (GFAP) and proliferating cell nuclear antigen (PCNA). Rr group revealed improved sucrose preference, increased undamaged neurons and decreased dark neurons. Moreover, Caspase 3 +ve cells were not detected, GFAP +ve cells increased and PCNA +ve cells were detected only in Rr group.
CONCLUSIONS
This work points to the role of Rr in depression improvement and in stimulation of NSCs proliferation.
MeSH Terms
Figure
Reference
-
References
1. Chen QG, Zeng YS, Qu ZQ, Tang JY, Qin YJ, Chung P, Wong R, Hägg U. The effects of Rhodiola rosea extract on 5-HT level, cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine. 2009. 16:830–838.
Article2. Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007. 102:1459–1465.
Article3. Taupin P. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent Nerv Syst Agents Med Chem. 2010. 10:16–21.
Article4. Brown RP, Gerbarg PL, Ramazanov Z. Rhodiola rosea: a phytomedicinal overview. Herbal Gram. 2002. 56:40–52.5. Dang H, Chen Y, Liu X, Wang Q, Wang L, Jia W, Wang Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009. 33:1417–1424.
Article6. Liu M, Hu J, Ma T, Wang S, Ding H. Application of a disposable screen-printed electrode to depression diagnosis for laboratory rats based on blood serotonin detection. Anal Sci. 2011. 27:839–843.
Article7. Venkataraman P, Selvakumar K, Krishnamoorthy G, Muthusami S, Rameshkumar R, Prakash S, Arunakaran J. Effect of melatonin on PCB (Aroclor 1254) induced neuronal damage and changes in Cu/Zn superoxide dismutase and glutathione peroxidase-4 mRNA expression in cerebral cortex, cerebellum and hippocampus of adult rats. Neurosci Res. 2010. 66:189–197.
Article8. Bancroft JD, Gamble MN. Theory and practice of histological techniques. 6th ed. London, Edinburgh, New York, Philadelphia, St Louis, Sydney and Toronto: Churchill-Livingstone;2007. 179.9. Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010. 19:237–270.
Article10. Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007. 80:1373–1381.
Article11. Garman RH. Histology of the central nervous system. Toxicol Pathol. 2011. 39:22–35.
Article12. Kherani ZS, Auer RN. Pharmacologic analysis of the mechanism of dark neuron production in cerebral cortex. Acta Neuropathol. 2008. 116:447–452.
Article13. Nowak B, Zadrożna M, Ossowska G, Sowa-Kućma M, Gruca P, Papp M, Dybała M, Pilc A, Nowak G. Alterations in hippocampal calcium-binding neurons induced by stress models of depression: a preliminary assessment. Pharmacol Rep. 2010. 62:1204–1210.
Article14. Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmström C, Panossian A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord J Psychiatry. 2007. 61:343–348.
Article15. Sima AA, Li ZG. The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes. 2005. 54:1497–1505.
Article16. Bachis A, Cruz MI, Nosheny RL, Mocchetti I. Chronic unpredictable stress promotes neuronal apoptosis in the cerebral cortex. Neurosci Lett. 2008. 442:104–108.
Article17. Sze CI, Lin YC, Lin YJ, Hsieh TH, Kuo YM, Lin CH. The role of glucocorticoid receptors in dexamethasone-induced apoptosis of neuroprogenitor cells in the hippocampus of rat pups. Mediators Inflamm. 2013. 2013:628094.
Article18. Palumbo DR, Occhiuto F, Spadaro F, Circosta C. Rhodiola rosea extract protects human cortical neurons against glutamate and hydrogen peroxide-induced cell death through reduction in the accumulation of intracellular calcium. Phytother Res. 2012. 26:878–883.
Article19. Robel S, Berninger B, Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011. 12:88–104.
Article20. Li LF, Yang J, Ma SP, Qu R. Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress- induced rat model of depression. Eur J Pharmacol. 2013. 711:42–49.
Article21. Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006. 15:407–421.
Article22. Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Fractalkine attenuates excito- neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem. 2011. 286:2308–2319.
Article23. Qu ZQ, Zhou Y, Zeng YS, Lin YK, Li Y, Zhong ZQ, Chan WY. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS One. 2012. 7:e29641.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of In Vivo Mobilization of Bone Marrow Stem Cells on the Pancreas of Diabetic Albino Rats (A Histological & Immunohistochemical Study)
- Possible Therapeutic Effect of Stem Cell in Atherosclerosis in Albino Rats. A Histological and Immunohistochemical Study
- The Possible Role of Mesenchymal Stem Cells Therapy in the Repair of Experimentally Induced Colitis in Male Albino Rats
- The Differential Developmental Neurotoxicity of Valproic Acid on Anterior and Posterior Neural Induction of Human Pluripotent Stem Cells
- Role of Bone Marrow Mesenchymal Stem Cells in the Treatment of CCL4 Induced Liver Fibrosis in Albino Rats: A Histological and Immunohistochemical Study