Dement Neurocogn Disord.
2014 Mar;13(1):1-6. 10.12779/dnd.2014.13.1.1.
Clinical Features and Prognostic Effects of Behavioral and Psychological Symptoms in Patients with Amyotrophic Lateral Sclerosis
- Affiliations
-
- 1Department of Neurology, College of Medicine, Hanyang University, Seoul, Korea. kimsh1@hanyang.ac.kr
- KMID: 1973683
- DOI: http://doi.org/10.12779/dnd.2014.13.1.1
Abstract
- BACKGROUND
The evaluation of behavioral and psychological symptoms (BPS) in ALS is important because its existence may serve as a prognostic factor and suggest a shared pathology with frontotemporal dementia (FTD) in ALS. In this study, we sought to identify the prevalence of the BPS of ALS patients and evaluate its relationship with the clinical profiles and survival of ALS patients.
METHODS
One hundred sixty-six patients were enrolled in a cross-sectional cohort analysis from September 2008 to February 2012. All patients had sporadic ALS without a genetic mutation and were collected clinical profiles. The t-test and chi-square test were used to assess differences in the clinical characteristics and caregiver-administered neuropsychiatric inventory (CGA-NPI) scores. The Kaplan-Meier method and Cox proportional hazard model were used for the survival analysis.
RESULTS
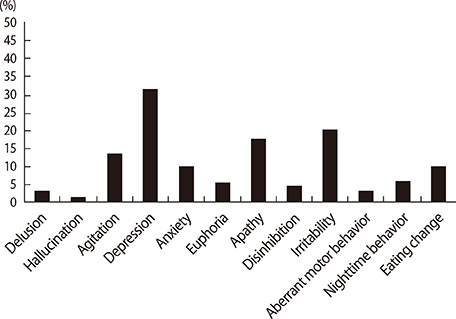
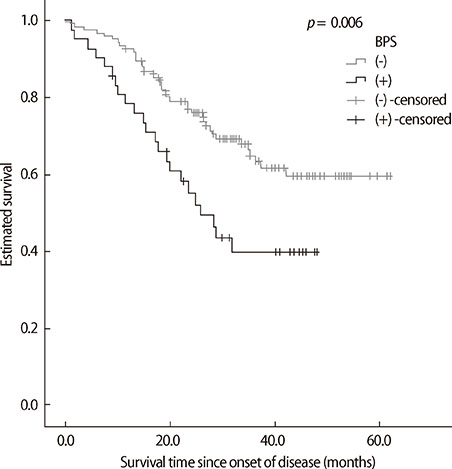
Forty-two patients had clinically significant BPS (42/166, 25.3%). ALS patients with BPS had worse clinical dementia rating (CDR), ALS Functional Rating Scale-Revised (ALSFRS-R) score, and progression rate of disease than those without BPS. Among CGA-NPI subscales, depression, irritability, apathy, and agitation were higher prevalent than the others. There was a trend for ALS patients with BPS having short survival time than those without BPS in the Kaplan-Meier analysis (p=0.006). However, in the Cox proportional hazard model, BPS in ALS patients were not associated with poor survival.
CONCLUSION
These results support the presence of an overlapping spectrum between ALS and FTD and emphasize the importance of neuropsychiatric evaluations in ALS. Although the association between BPS and prognosis are not explained clearly, these results could be used to stratify ALS patients according to neuropsychiatric symptoms and help investigators to evaluate the BPS in ALS patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Consonni M, Iannaccone S, Cerami C, Frasson P, Lacerenza M, Lunetta C, et al. The cognitive and behavioural profile of amyotrophic lateral sclerosis: application of the consensus criteria. Behav Neurol. 2013; 27:143–153.
Article2. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007; 6:994–1003.3. Baek WK, Park A, Kim HY, Kim SH. Amyotrophic Lateral Sclerosis in Korea: Clinical Characteristics and Prognostic Factors. J Korean Neurol Assoc. 2011; 29:16–24.4. Oh SI, Park A, Kim HJ, Oh KW, Choi H, Kwon MJ, et al. Spectrum of Cognitive Impairment in Korean ALS Patients without Known Genetic Mutations. PLoS One. 2014; 9:e87163.5. Lillo P, Mioshi E, Zoing MC, Kiernan MC, Hodges JR. How common are behavioural changes in amyotrophic lateral sclerosis? Amyotroph Lateral Scler. 2011; 12:45–51.6. Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013; 12:368–380.
Article7. Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013; 14:248–264.
Article8. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1:293–299.
Article9. Jang JH, Kwon MJ, Choi WJ, Oh KW, Koh SH, Ki CS, et al. Analysis of the C9orf72 hexanucleotide repeat expansion in Korean patients with familial and sporadic amyotrophic lateral sclerosis. Neurobiol Aging. 2013; 34:1311e1317–e1319.
Article10. Kwon MJ, Baek W, Ki CS, Kim HY, Koh SH, Kim JW, et al. Screening of the SOD1, FUS, TARDBP, ANG, and OPTN mutations in Korean patients with familial and sporadic ALS. Neurobiol Aging. 2012; 33:1017e1017–e1023.
Article11. Kang SJ, Choi SH, Lee BH, Jeong Y, Hahm DS, Han IW, et al. Caregiver-Administered Neuropsychiatric Inventory (CGA-NPI). J Geriatr Psychiatry Neurol. 2004; 17:32–35.
Article12. Chin J, Seo SW, Kim SH, Park A, Ahn HJ, Lee BH, et al. Neurobehavioral dysfunction in patients with subcortical vascular mild cognitive impairment and subcortical vascular dementia. Clin Neuropsychol. 2012; 26:224–238.
Article13. Kang Y. A normative study of the Korean-Mini Mental State Examination (K-MMSE) in the elderly. Korean J Psychol. 2006; 25:1–12.
Article14. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43:2412–2414.
Article15. Gordon PH, Miller RG, Moore DH. Alsfrs-R. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004; 5:Suppl 1. 90–93.
Article16. Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006; 66:265–267.
Article17. Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009; 10:131–146.
Article18. Cooper-Knock J, Shaw PJ, Kirby J. The widening spectrum of C9ORF72-related disease; genotype/phenotype correlations and potential modifiers of clinical phenotype. Acta Neuropathol. 2014; 127:333–345.
Article19. Kertesz A, Ang LC, Jesso S, MacKinley J, Baker M, Brown P, et al. Psychosis and hallucinations in frontotemporal dementia with the C9ORF72 mutation: a detailed clinical cohort. Cogn Behav Neurol. 2013; 26:146–154.
Article20. Raaphorst J, Beeldman E, De Visser M, De Haan RJ, Schmand B. A systematic review of behavioural changes in motor neuron disease. Amyotroph Lateral Scler. 2012; 13:493–501.
Article21. Kim EJ, Kwon JC, Park KH, Park KW, Lee JH, Choi SH, et al. Clinical and genetic analysis of MAPT, GRN, and C9orf72 genes in Korean patients with frontotemporal dementia. Neurobiol Aging. 2014; 35:1213e1213–e1217.
Article22. Lillo P, Savage S, Mioshi E, Kiernan MC, Hodges JR. Amyotrophic lateral sclerosis and frontotemporal dementia: A behavioural and cognitive continuum. Amyotroph Lateral Scler. 2012; 13:102–109.
Article23. Raaphorst J, Beeldman E, Schmand B, Berkhout J, Linssen WH, van den Berg LH, et al. The ALS-FTD-Q: a new screening tool for behavioral disturbances in ALS. Neurology. 2012; 79:1377–1383.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evolving Diagnostic Criteria in Amyotrophic Lateral Sclerosis and Its Differential Diagnosis
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Syndrome of Progressive Bulbar Palsy in Amyotrophic Lateral Sclerosis: A Case Report
- Apraxia of Eyelid Closure and Motor Impersistence of Eyelid in a Patient with Amyotrophic Lateral Sclerosis
- Amyotrophic Lateral Sclerosis in Korea: Clinical Characteristics and Prognostic Factors