Cancer Res Treat.
2009 Dec;41(4):196-204.
Docetaxel versus Paclitaxel Combined with 5-FU and Leucovorin in Advanced Gastric Cancer: Combined Analysis of Two Phase II Trials
- Affiliations
-
- 1Department of Medical Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. jeunghc@yuhs.ac
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
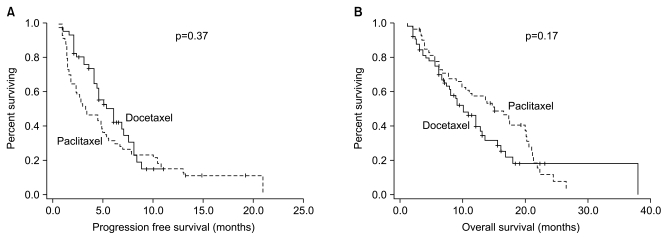
This is an ad hoc analysis of two phase II studies which compared the efficacy and safety of two taxanes (paclitaxel and docetaxel) combined with 5-fluorouracil (5-FU) and leucovorin (LV) in advanced gastric cancer. MATERIALS AND METHODS: Patients with advanced gastric adenocarcinoma who were untreated or had only received first-line chemotherapy, were treated with either paclitaxel (PFL; 175 mg/m2) or docetaxel (DFL; 75 mg/m2) on day 1, followed by a bolus of LV (20 mg/m2 days 1~3) and a 24-hour infusion of 5-FU (1,000 mg/m2 days 1~3) every 3 weeks. The primary endpoint was overall response rate (ORR) and the secondary endpoint included survival and toxicity. RESULTS: Sixty-six patients received DFL (first-line [n=38]; and second-line [n=28]) and 60 patients received PFL (first-line [n=37]; and second-line [n=23]). The ORRs were not significantly different between the 2 groups (DFL, 26%; PFL, 38%). With a median follow-up of 9.5 months, the progression free survival was 5.2 months (95% confidence interval [CI], 4.2~6.5 months) for DFL and 3.3 months (95% CI, 1.3~5.5 months) for PFL (p=0.17). The overall survival was also comparable between the patients who received DFL and PFL (10.0 months [95% CI, 7.2~12.5 months] and 13.9 months [95% CI, 10.9~19.2 months], respectively; p=0.37). The most frequent grade 3~4 adverse event was neutropenia (DFL, 71%; PFL, 62%). DFL and PFL had different non-hematologic toxicities; specifically, grade > or =3 mucositis (5%) and diarrhea (3%) were common in DFL, while nausea/vomiting (15%) and peripheral neuropathy (5%) were common in PFL. CONCLUSION: Thus, the two taxanes had similar efficacy in the treatment of advanced gastric cancer, but different toxicity profiles. Prospective comparative studies are required to further clarify the role of taxanes in the treatment of advanced gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee JA, Yoon SS, Yang SH, Kim S, Heo DS, Bang YG, et al. FAM versus etoposide, adriamycin, and cisplatin: a random assignment trial in advanced gastric cancer. J Korean Cancer Assoc. 1993; 25:461–467.2. Thuss-Patience PC, Kretzschmar A, Repp M, Kingreen D, Hennesser D, Micheel S, et al. Docetaxel and continuous-infusion fluorouracil versus epirubicin, cisplatin, and fluorouracil for advanced gastric adenocarcinoma: a randomized phase II study. J Clin Oncol. 2005; 23:494–501. PMID: 15659494.
Article3. Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999; 85:295–301. PMID: 10023695.
Article4. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999; 22:580–586. PMID: 10597742.5. Bokemeyer C, Lampe CS, Clemens MR, Hartmann JT, Quietzsch D, Forkmann L, et al. A phase II trial of paclitaxel and weekly 24 h infusion of 5-fluorouracil/folinic acid in patients with advanced gastric cancer. Anticancer Drugs. 1997; 8:396–399. PMID: 9180395.
Article6. Jung JJ, Jeung HC, Chung HC, Lee JO, Kim TS, Kim YT, et al. In vitro pharmacogenomic database and chemosensitivity predictive genes in gastric cancer. Genomics. 2009; 93:52–61. PMID: 18804159.
Article7. Jeung HC, Rha SY, Kim YT, Noh SH, Roh JK, Chung HC. A phase II study of infusional 5-fluorouracil and low-dose leucovorin with docetaxel for advanced gastric cancer. Oncology. 2006; 70:63–70. PMID: 16446551.
Article8. Im CK, Jeung HC, Rha SY, Yoo NC, Noh SH, Roh JK, et al. A phase II study of paclitaxel combined with infusional 5-fluorouracil and low-dose leucovorin for advanced gastric cancer. Cancer Chemother Pharmacol. 2008; 61:315–321. PMID: 18026677.
Article9. Rigas JR. Taxane-platinum combinations in advanced non-small cell lung cancer: a review. Oncologist. 2004; 9:16–23. PMID: 15161987.
Article10. Esteban E, Gonzalez de, Fernandez Y, Corral N, Fra J, Muniz I, et al. Prospective randomised phase II study of docetaxel versus paclitaxel administered weekly in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Ann Oncol. 2003; 14:1640–1647. PMID: 14581272.
Article11. Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L, Lower E, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005; 23:5542–5551. PMID: 16110015.
Article12. Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004; 96:1682–1691. PMID: 15547181.
Article13. Imamura H, IIishi H, Tsuburaya A, Hatake K, Imamoto H, Esaki T, et al. Randomized phase III study of irinotecan plus S-1 (IRIS) versus S-1 alone as first-line treatment for advanced gastric cancer. ASCO Meeting Proc. 2007; 25:S18.14. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9:215–221. PMID: 18282805.
Article15. Van Cutsem E, Moiseyenko V, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–4997. PMID: 17075117.
Article16. Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs. 2006; 17:225–229. PMID: 16428942.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Advanced Gastric Cancer: Slow but Further Progress
- A New Option for Advanced Gastric Cancer: Docetaxel and Novel Oral Fluoropyrimidine Combination Chemotherapy
- FOLFOX-4 Combination Chemotherapy as a First-line Treatment in Patients with Advanced Gastric Cancer
- A Phase II Study of Leucovorin, 5-FU and Docetaxel Combination Chemotherapy in Patients with Inoperable or Postoperative Relapsed Gastric Cancer
- A Phase II Trial of Paclitaxel, 5-fluorouracil (5-FU) and Cisplatin in Patients with Metastatic or Recurrent Gastric Cancer