J Korean Endocr Soc.
2008 Dec;23(6):395-403. 10.3803/jkes.2008.23.6.395.
Discrepancy between the Growth Hormone and Insulin-like Growth Factor-I Concentrations in Patients with Acromegaly
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 2The Rusk Memorial Medical Center, Korea.
- KMID: 1965992
- DOI: http://doi.org/10.3803/jkes.2008.23.6.395
Abstract
-
BACKGROUND: This study was performed to evaluate the frequency and clinical characteristics of patients with active acromegaly and who show discordance of the growth hormone (GH) level and the insulin-like growth factor-I (IGF-I) level.
METHODS
We reviewed the medical records of the patients who were diagnosed with acromegaly between 01/01/1995 and 6/30/2007 at Seoul National University Hospital. We selected only the patients whose basal GH and IGF-I levels were available. We investigated the pre- and post-operative clinical characteristics, as well as the blood concentrations of GH and IGF-I. The concordance rate between the two hormones was examined. The patients were considered to have active disease on the basis of their IGF-I levels above the normal range, after adjustment for age and gender, and their mean basal GH value was > or = 2.5 microgram/L. The hormone levels and the clinical parameters were compared between the hormone concordant and discordant groups.
RESULTS
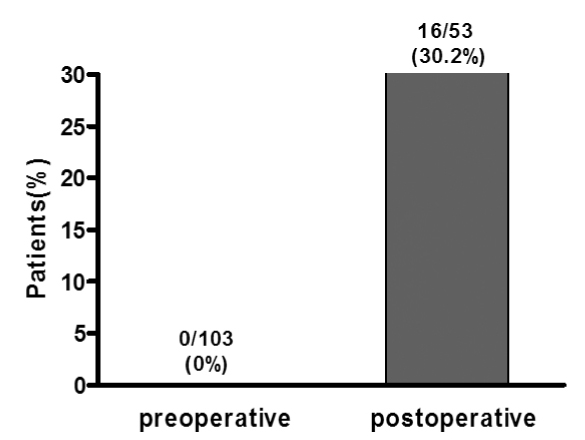
We reviewed the preoperative records of 103 acromegalic patients, and these patients met the above-mentioned criteria. 53 postoperative patients who were not cured by operation were monitored without them receiving radiation or medical therapy. Both the basal GH and IGF-I levels were above normal in 103 patients preoperatively, and the discordant rate was 0% (0/103 cases). Postoperatively, the discordant rate between the two hormones was increased to 30.2% (16/53 cases). Age, gender, body mass index and tumor size were insignificantly different between the concordant and discordant groups. However, postoperative residual tumors were less frequently observed in the discordant group (P = 0.006).
CONCLUSION
For the patients with acromegaly, unlike the 0% discordance preoperatively, 30.2% of patients showed a discrepancy between their GH and IGF-I levels postoperatively. The patients who had hormonal discrepancy were less likely to have residual tumors after operation. Considering the frequency of this hormonal discrepancy, both hormone levels should be measured to evaluate the disease activity after treatment. Further, oral glucose tolerance testing should be performed and especially for the patients with an increased GH level, but who have a normal IGF-I concentration.
MeSH Terms
Figure
Reference
-
1. Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf). 1994. 41:95–102.2. Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000. 85:526–529.3. Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998. 83:2730–2734.4. Bates AS, Van't Hoff W, Jones JM, Clayton RN. An audit of outcome of treatment in acromegaly. Q J Med. 1993. 86:293–299.5. Growth Hormone Research Society. Pituitary Society. Biochemical assessment and long-term monitoring in patients with acromegaly: statement from a joint consensus conference of the Growth Hormone Research Society and the Pituitary Society. J Clin Endocrinol Metab. 2004. 89:3099–3102.6. Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989. 10:68–91.7. Clemmons DR, Van Wyk JJ, Ridgway EC, Kliman B, Kjellberg RN, Underwood LE. Evaluation of acromegaly by radioimmunoassay of somatomedin-C. N Engl J Med. 1979. 301:1138–1142.8. Bates AS, Evans AJ, Jones P, Clayton RN. Assessment of GH status in acromegaly using serum growth hormone, serum insulin-like growth factor-1 and urinary growth hormone excretion. Clin Endocrinol (Oxf). 1995. 42:417–423.9. Costa AC, Rossi A, Martinelli CE Jr, Machado HR, Moreira AC. Assessment of disease activity in treated acromegalic patients using a sensitive GH assay: should we achieve strict normal GH levels for a biochemical cure? J Clin Endocrinol Metab. 2002. 87:3142–3147.10. Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004. 89:667–674.11. Kauppinen-Makelin R, Sane T, Reunanen A, Valimaki MJ, Niskanen L, Markkanen H, Loyttyniemi E, Ebeling T, Jaatinen P, Laine H, Nuutila P, Salmela P, Salmi J, Stenman UH, Viikari J, Voutilainen E. A nationwide survey of mortality in acromegaly. J Clin Endocrinol Metab. 2005. 90:4081–4086.12. Dimaraki EV, Jaffe CA, DeMott-Friberg R, Chandler WF, Barkan AL. Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J Clin Endocrinol Metab. 2002. 87:3537–3542.13. Espinosa-de-Los-Monteros AL, Sosa E, Cheng S, Ochoa R, Sandoval C, Guinto G, Mendoza V, Hernandez I, Molina M, Mercado M. Biochemical evaluation of disease activity after pituitary surgery in acromegaly: a critical analysis of patients who spontaneously change disease status. Clin Endocrinol (Oxf). 2006. 64:245–249.14. Dobrashian RD, O'Halloran DJ, Hunt A, Beardwell CG, Shalet SM. Relationships between insulin-like growth factor-1 levels and growth hormone concentrations during diurnal profiles and following oral glucose in acromegaly. Clin Endocrinol (Oxf). 1993. 38:589–593.15. Freda PU, Nuruzzaman AT, Reyes CM, Sundeen RE, Post KD. Significance of "abnormal" nadir growth hormone levels after oral glucose in postoperative patients with acromegaly in remission with normal insulin-like growth factor-I levels. J Clin Endocrinol Metab. 2004. 89:495–500.16. Minuto F, Resmini E, Boschetti M, Arvigo M, Sormani MP, Giusti M, Ferone D, Barreca A. Assessment of disease activity in acromegaly by means of a single blood sample: comparison of the 120th minute postglucose value with spontaneous GH secretion and with the IGF system. Clin Endocrinol (Oxf). 2004. 61:138–144.17. Ayuk J, Clayton RN, Holder G, Sheppard MC, Stewart PM, Bates AS. Growth hormone and pituitary radiotherapy, but not serum insulin-like growth factor-I concentrations, predict excess mortality in patients with acromegaly. J Clin Endocrinol Metab. 2004. 89:1613–1617.18. Kaltsas GA, Isidori AM, Florakis D, Trainer PJ, Camacho-Hubner C, Afshar F, Sabin I, Jenkins JP, Chew SL, Monson JP, Besser GM, Grossman AB. Predictors of the outcome of surgical treatment in acromegaly and the value of the mean growth hormone day curve in assessing postoperative disease activity. J Clin Endocrinol Metab. 2001. 86:1645–1652.19. Damjanovic SS, Neskovic AN, Petakov MS, Popovic V, Macut D, Vukojevic P, Joksimovic MM. Clinical indicators of biochemical remission in acromegaly: does incomplete disease control always mean therapeutic failure? Clin Endocrinol (Oxf). 2005. 62:410–417.20. Alexopoulou O, Bex M, Abs R, T'sjoen G, Velkeniers B, Maiter D. Divergence between growth hormone and insulin-like growth factor-I concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab. 2008. 93:1324–1330.21. Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab. 1998. 83:3411–3418.22. Daughaday WH, Starkey RH, Saltman S, Gavin JR 3rd, Mills-Dunlap B, Heath-Monnig E. Characterization of serum growth hormone (GH) and insulin-like growth factor I in active acromegaly with minimal elevation of serum GH. J Clin Endocrinol Metab. 1987. 65:617–623.23. Ho KY, Weissberger AJ. Characterization of 24-hour growth hormone secretion in acromegaly: implications for diagnosis and therapy. Clin Endocrinol (Oxf). 1994. 41:75–83.24. Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM. The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab. 2001. 86:259–266.25. Colao A, Pivonello R, Cavallo LM, Gaccione M, Auriemma RS, Esposito F, Cappabianca P, Lombardi G. Age changes the diagnostic accuracy of mean profile and nadir growth hormone levels after oral glucose in postoperative patients with acromegaly. Clin Endocrinol (Oxf). 2006. 65:250–256.26. Meinhardt UJ, Ho KK. Modulation of growth hormone action by sex steroids. Clin Endocrinol (Oxf). 2006. 65(4):413–422.27. Soliman AT, Hassan AE, Aref MK, Hintz RL, Rosenfeld RG, Rogol AD. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr Res. 1986. 20:1122–1130.28. Powell DR, Rosenfeld RG, Baker BK, Liu F, Hintz RL. Serum somatomedin levels in adults with chronic renal failure: the importance of measuring insulin-like growth factor I (IGF-I) and IGF-II in acid-chromatographed uremic serum. J Clin Endocrinol Metab. 1986. 63:1186–1192.29. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995. 16:3–34.30. Bevan JS, Atkin SL, Atkinson AB, Bouloux PM, Hanna F, Harris PE, James RA, McConnell M, Roberts GA, Scanlon MF, Stewart PM, Teasdale E, Turner HE, Wass JA, Wardlaw JM. Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor size. J Clin Endocrinol Metab. 2002. 87:4554–4563.31. Lorcy Y, Dejager S, Chanson P. French Octerotide LAR Group. Time course of GH and IGF-1 levels following withdrawal of long-acting octreotide in acromegaly. Pituitary. 2000. 3:193–197.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship of Insulin like Growth Factor I with Pharmacologically Stimulated Growth Hormone Secretion in Growth Hormone Deficient Children
- The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin
- A Case of Acromegaly Presenting with Diabetic Ketoacidosis
- A Case of Acromegaly with Gall Bladder Cancer
- A Case of Acromegaly Associated with Lung and Gastric Cancer