J Korean Med Assoc.
2014 Nov;57(11):919-926. 10.5124/jkma.2014.57.11.919.
Medical technology development and globalization: the role of the medical device industry
- Affiliations
-
- 1Corporate Affairs, Medtronic Korea, Seoul, Korea.
- KMID: 1958392
- DOI: http://doi.org/10.5124/jkma.2014.57.11.919
Abstract
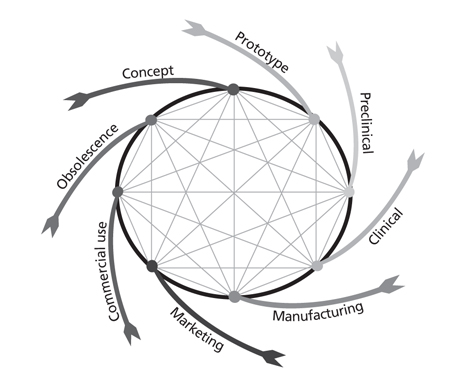
- Modern medical technology aligns with medical device advancement, and new medical devices create the need for new procedure techniques. Unmet needs that physicians experience in clinical practice suggest solutions to problems; to solve these, collaboration between medical device manufacturers and physicians becomes a starting point for new medical device development. Commercialization of medical devices is greatly impacted by the product regulatory approval system, health technology assessment, and reimbursement system. Speed to market plays a far greater role in the medical device market than in the pharmaceutical market. Considering the current trend of evidence-based medicine and value-based pricing, efforts to generate clinical evidence should be strengthened even further while a greater focus should be placed on efforts to introduce global multicenter pre-market clinical trials in the Republic of Korea through strong collaboration with global companies. Since the strength and quality of clinical evidence is comparatively low in medical device studies, this could affect the decision making process and raise the issue of uncertainty. To overcome this issue, a risk-sharing system in the medical device field and 'coverage with evidence development' should be introduced; by doing so, evidence generation opportunities can be created without burying innovative technology and solving the issue of uncertain decisions. In addition, reimbursement coverage to support the costs of clinical studies needs to be established for early evidence generation, as seen in other countries.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Managing health technologies
Tae-Hwan Lim
J Korean Med Assoc. 2014;57(11):904-905. doi: 10.5124/jkma.2014.57.11.904.Foreign direct investment of Covidien Center of Innovation in medical device industry
Sang-Soo Lee, Moo-Yeol Lee
J Korean Med Assoc. 2015;58(10):933-941. doi: 10.5124/jkma.2015.58.10.933.
Reference
-
1. Chatterji AK, Fabrizio KR, Mitchell W, Schulman KA. Physi-cian-industry cooperation in the medical device industry. Health Aff (Millwood). 2008; 27:1532–1543.
Article2. Ministry of Health and Welfare. Mid-/long-term development plan for medical device industry aiming at global 7th rank by 2020 [Internet]. Sejong: Ministry of Health and Welfare;2014. cited 2014 Nov 3. Available from: http://www.mw.go.kr/front_new/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&CONT_SEQ=299308&page=1.3. Ministry of Food and Drug Safety. Report on production, export and import of medical device in 2013. Cheongju: Mini-stry of Food and Drug Safety;2013.4. Korea Medical Devices Industry Association. Position paper. Seoul: Korea Medical Devices Industry Association;2013.5. US Food and Drug Administration. Medical device inno-vation initiative white paper: CDRH Innovation Initiative [Internet]. Silver Spring: US Food and Drug Administration;2011. cited 2014 Nov 3. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHInnovation/ucm242067.htm.6. Kim SB. Business performance analysis of medical device companies by countries in 2012. KHIDI Brief. 2013; 75:[Epub]. http://info.khidi.or.kr/www/download.jsp?i=5429.7. Seo GS. Analysis on market size by medical device sectors and R&D investment status. KHIDI Brief. 2012; 20:[Epub]. http://medicaldevice.khidi.or.kr/board.es?mid=a10401000000&bid=0004&act=view&list_no=457.8. Ernst & Young Global Limited. Pulse of the industry: medical technology report 2013 [Internet]. London: Ernst & Young Global Limited;2013. cited 2014 Nov 3. Available from: http://www.ey.com/US/en/Industries/Life-Sciences/Pulse-of-the-industry---medical-technology-report-2013.9. Korea Medical Devices Industry Association. The proposal to Ministry of Health and Welfare about medical device clinical research policy. Seoul: Korea Medical Devices Industry Association;2012.10. Medicare Program; Criteria and Procedures for Extending Coverage to Certain Devices and Related Services, 42 C.F.R. 405. 1995.11. Public Health Code Article, L 1121-16-1. 2011.12. Hospital Remuneration Act, KHEntgG, No. 8. 2002.13. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gud-nason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK. TASTE Trial. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013; 369:1587–1597.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Foreign direct investment of Covidien Center of Innovation in medical device industry
- Survey for Government Policies Regarding Strategies for the Commercialization and Globalization of Digital Therapeutics
- The IT Industry’s Influence on Next-Generation Aviation Technologies
- Medical device clinical trial
- Medical Applications of 3D Printing and Standardization Issues