Infect Chemother.
2011 Aug;43(4):372-376. 10.3947/ic.2011.43.4.372.
Native Valve Infective Endocarditis due to Staphylococcus lugdunensis Confirmed by 16S Ribosomal RNA Sequencing
- Affiliations
-
- 1Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. krpeck@skku.edu
- 2Department of Infectious Disease, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Molecular Cell Biology, Samsung Biomedical Research Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1936077
- DOI: http://doi.org/10.3947/ic.2011.43.4.372
Abstract
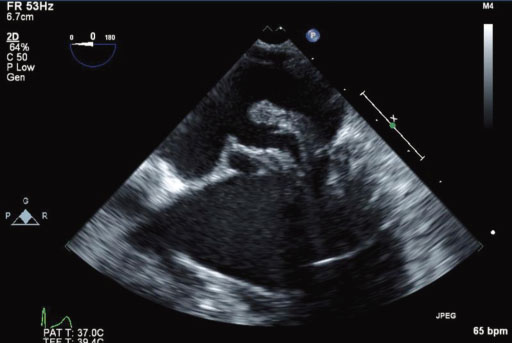
- Staphylococcus lugdunensis is a Gram-positive, coagulase-negative Staphylococcus (CNS) species that is found as a skin commensal and has been implicated in fulminant invasive diseases such as infective endocarditis. S. lugudunensis infections resemble Staphylococcus aureus infections in terms of virulence, tissue destruction and clinical course. Although correct identification and determination of the susceptibility profile are important, some commercial systems may misidentify S. lugdunensis. We report a case of native valve infective endocarditis caused by S. lugdunensis, which was misidentified by the Vitek 2 system but identified correctly by 16S ribosomal RNA (rRNA) gene sequencing in a 72-year-old male patient. The patient had multiple vegetations on his mitral valve, and the largest one was found on the posterior mitral valve leaflet. It was 2.5 cm in size and hypermobile. Diffuse valvular abscess was also observed. He had persistent bacteremia for appoximately 8 days, which was resolved after immediate surgery and antibiotic therapy. When a patient with severe sepsis syndrome grows S. aureus or CNS other than S. lugdunensis on a commercial automatic culture system, the possibility of S. lugdunensis should be considered and further confirmatory testing such as 16S rRNA sequencing may be very useful.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Native Valve Endocarditis due to Corynebacterium striatum confirmed by 16S Ribosomal RNA Sequencing: A Case Report and Literature Review
Hyo-Lim Hong, Hwi-In Koh, A-Jin Lee
Infect Chemother. 2016;48(3):239-245. doi: 10.3947/ic.2016.48.3.239.
Reference
-
1. Anguera I, Del Río A, Miró JM, Matínez-Lacasa X, Marco F, Gumá JR, Quaglio G, Claramonte X, Moreno A, Mestres CA, Mauri E, Azqueta M, Benito N, García-de la María C, Almela M, Jiménez-Expósito MJ, Sued O, De Lazzari E, Gatell JM. Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005. 91:e10.2. Seenivasan MH, Yu VL. Staphylococcus lugdunensis endocarditis--the hidden peril of coagulase-negative staphylococcus in blood cultures. Eur J Clin Microbiol Infect Dis. 2003. 22:489–491.
Article3. Cho HJ, Seol SH, Park SY, Jun HS, Kim DK, Kim DI, Kim DS. A case of native valve infective endocarditis caused by Staphylococcus lugdunensis. Korean J Med. 2011. 80:212–215.4. Choi SH, Park HG, Byun SW, Koo DH, Kang HS, Jang HJ, Kim YS, Woo JH, Kim YH, Choi SH. A case of infective endocarditis due to Staphylococcus lugdunensis. Infect Chemother. 2006. 38:277–281.5. von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002. 2:677–685.
Article6. Mateo M, Maestre JR, Aguilar L, Cafini F, Puente P, Sánchez P, Alou L, Giménez MJ, Prieto J. Genotypic versus phenotypic characterization, with respect to susceptibility and identification, of 17 clinical isolates of Staphylococcus lugdunensis. J Antimicrob Chemother. 2005. 56:287–291.
Article7. Sohn KM, Ko KS, Kim J, Rhee JY, Oh WS, Peck KR, Song JH. Identification of Gemella species by 16S ribosomal RNA gene sequencing from two patients with infective endocarditis. Korean J Med. 2006. 70:591–596.8. Zhu XY, Zhong T, Pandya Y, Joerger RD. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl Environ Microbiol. 2002. 68:124–137.
Article9. Ebright JR, Penugonda N, Brown W. Clinical experience with Staphylococcus lugdunensis bacteremia: a retrospective analysis. Diagn Microbiol Infect Dis. 2004. 48:17–21.
Article10. De Paulis AN, Predari SC, Chazarreta CD, Santoianni JE. Five-test simple scheme for species-level identification of clinically significant coagulase-negative staphylococci. J Clin Microbiol. 2003. 41:1219–1224.
Article11. Zinkernagel AS, Zinkernagel MS, Elzi MV, Genoni M, Gubler J, Zbinden R, Mueller NJ. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection. 2008. 36:314–321.
Article12. Choi SH, Chung JW, Lee EJ, Kim TH, Lee MS, Kang JM, Song EH, Jun JB, Kim MN, Kim YS, Woo JH, Choi SH. Incidence, characteristics, and outcomes of Staphylococcus lugdunensis bacteremia. J Clin Microbiol. 2010. 48:3346–3349.
Article13. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 16th informational supplement. Document M100-S16. 2007. Wayne, PA: CLSI.14. Leung MJ, Nuttall N, Pryce TM, Coombs GW, Pearman JW. Colony variation in Staphylococcus lugdunensis. J Clin Microbiol. 1998. 36:3096–3098.15. Hussain Z, Stoakes L, Massey V, Diagre D, Fitzgerald V, El Sayed S, Lannigan R. Correlation of oxacillin MIC with mecA gene carriage in coagulase-negative staphylococci. J Clin Microbiol. 2000. 38:752–754.
Article16. Zadoks RN, Watts JL. Species identification of coagulase-negative staphylococci: genotyping is superior to phenotyping. Vet Microbiol. 2009. 134:20–28.
Article17. Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol. 2007. 45:2761–2764.
Article18. Woo PC, Lau SK, Teng JL, Tse H, Yuen KY. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008. 14:908–934.
Article19. Bosshard PP, Kronenberg A, Zbinden R, Ruef C, Böttger EC, Altwegg M. Etiologic diagnosis of infective endocarditis by broad-range polymerase chain reaction: a 3-year experience. Clin Infect Dis. 2003. 37:167–172.
Article20. Pada S, Lye DC, Leo YS, Barkham T. Utility of 16S ribosomal DNA sequencing in the diagnosis of Staphylococcus lugdunensis native valve infective endocarditis: case report and literature review. Int J Infect Dis. 2009. 13:e511–e513.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Native Valve Endocarditis due to Corynebacterium striatum confirmed by 16S Ribosomal RNA Sequencing: A Case Report and Literature Review
- A Case of Native Valve Infective Endocarditis Caused by Staphylococcus lugdunensis
- A Case of Infective Endocarditis due to Staphylococcus lugdunensis
- A Case of Pulmonary Artery Endarteritis due to Staphylococcus lugdunensis in Patient with Clinically Silent Patent Ductus Arteriosus
- First Case of Bartonella quintana Endocarditis in Korea