Korean J Phys Anthropol.
2014 Jun;27(2):71-77. 10.11637/kjpa.2014.27.2.71.
Immunohistochemical Study of O-GlcNAcylation in Human Skin Tumors
- Affiliations
-
- 1Department of Dermatology and Research Institute for Medical Sciences, School of Medicine, Chungnam National University, Daejeon, Korea. cdkimd@cnu.ac.kr
- 2Department of Anatomy, School of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 1917126
- DOI: http://doi.org/10.11637/kjpa.2014.27.2.71
Abstract
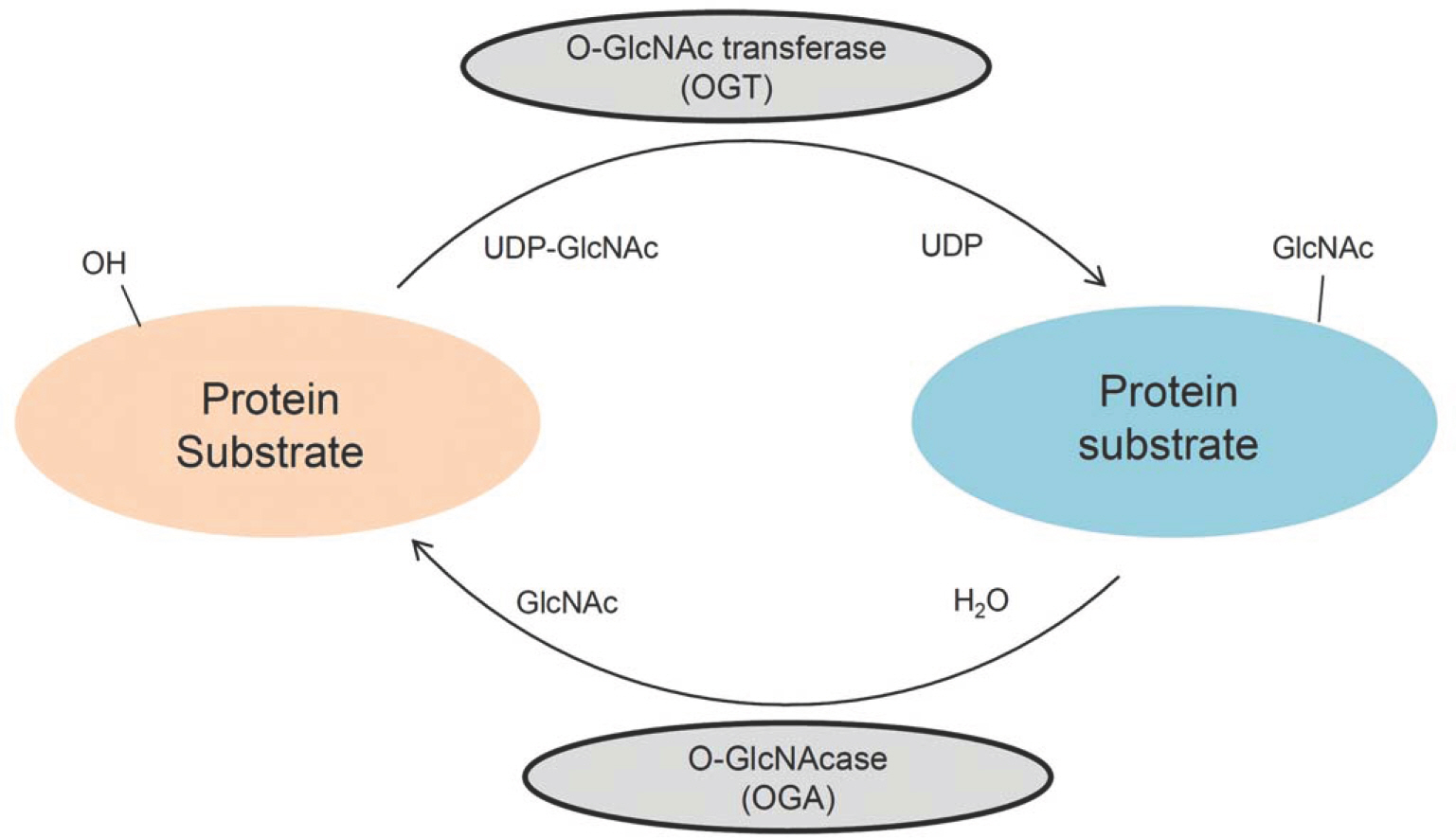
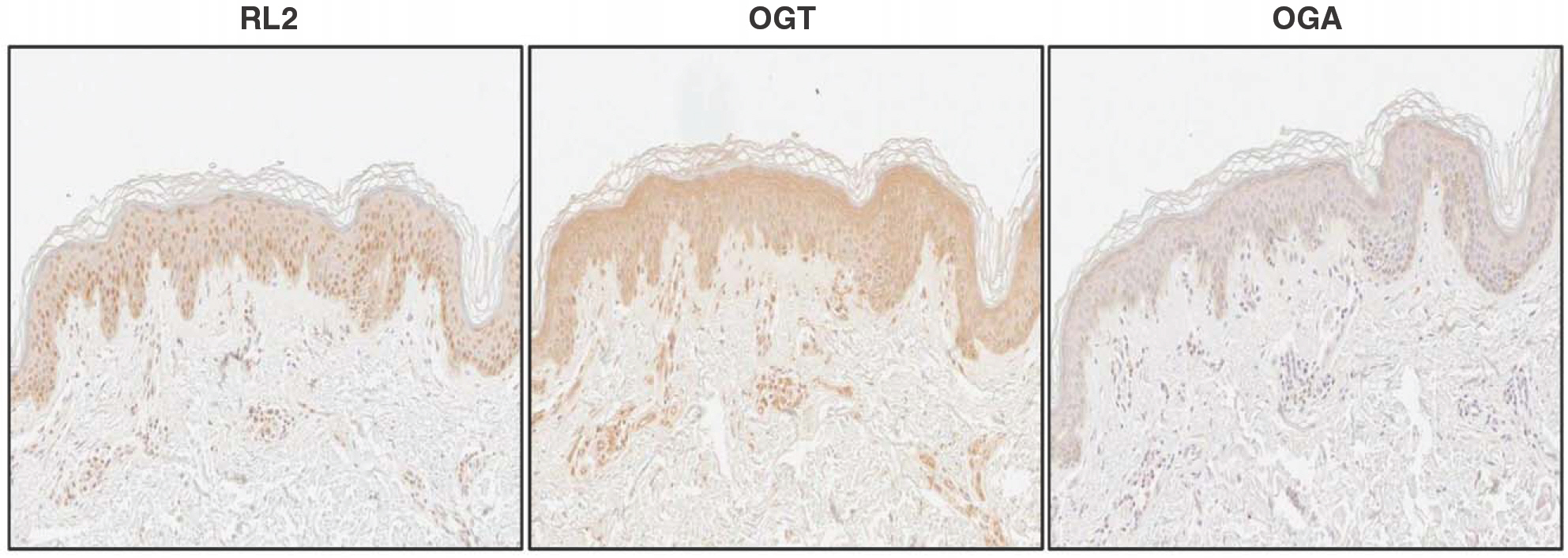
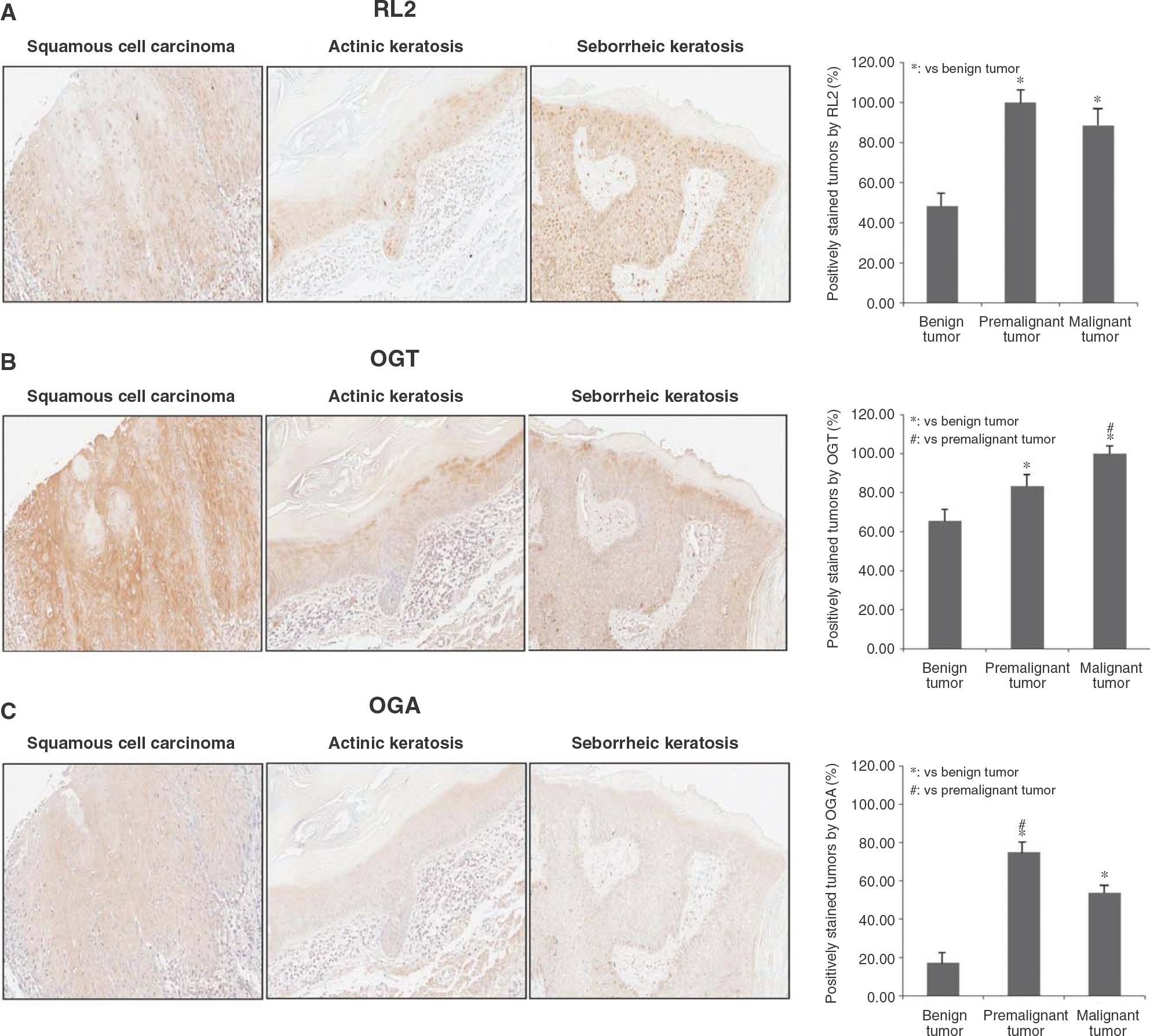
- O-linked beta-N-acetylglucosamine modification is an important post-translational modification, emerging as a novel regulatory mechanism in various cellular events. Recently, several studies have shown that O-GlcNAcylation plays an essential role in human breast, lung, and colon cancers. With regard to skin cancers, the role of O-GlcNAcylation has yet to be elucidated. To investigate whether O-GlcNAcylation is linked to human skin tumor development, immunohistochemical analysis was performed to investigate the presence of O-GlcNAcylation in various skin tumors. We evaluated the levels of O-GlcNAcylation, O-GlcNAc transferase, and O-GlcNAcase in 29 benign tumors, 12 premalignant tumors, and 26 malignant tumors in skin. Compared to the benign tumors, premalignant and malignant tumors had increased patterns of O-GlcNAcylation. In addition, the O-GlcNAc transferase and O-GlcNAcase levels were higher in premalignant and malignant tumors than in benign tumors. Interestingly, O-GlcNAcase levels were significantly increased in premalignant tumors compared to benign and malignant tumors. These results suggest that O-GlcNAcylation of proteins may play an important role in the development of human skin tumors.
MeSH Terms
Figure
Reference
-
References
1. Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984; 259:3308–17.
Article2. Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007; 446:1017–22.3. Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997; 272:9308–15.4. Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglu-cosaminidase from human brain. J Biol Chem. 2001; 276:9838–45.5. Kang JG, Park SY, Ji S, Jang I, Park S, Kim HS, et al. O-GlcNAc protein modification in cancer cells increases in response to glucose deprivation through glycogen degradation. J Biol Chem. 2009; 284:34777–84.
Article6. Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, et al. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010; 70:6344–51.
Article7. Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, et al. O-Glc-NAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011; 1812:514–9.
Article8. Snow CM, Senior A, Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987; 104:1143–56.
Article9. Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010; 29:2831–42.
Article10. Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked β-N-acetylglu-cosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012; 287:11070–81.
Article11. Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-β-D-glucosaminidase silencing on cell phenotype and transcriptome. J Biol Chem. 2012; 287:28755–69.
Article12. Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, et al. O-Glc-NAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol. 2012; 29:985–93.
Article13. Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem. 2013; 288:15121–30.
Article14. Vosseller K, Sakabe K, Wells L, Hart GW. Diverse regulation of protein function by O-GlcNAc: a nuclear and cytoplasmic carbohydrate post-translational modification. Curr Opin Chem Biol. 2002; 6:851–7.
Article15. Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012; 13:312–21.16. Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010; 35:547–55.
Article17. Ma Z, Vosseller K. O-GlcNAc in cancer biology. Amino Acids. 2013; 45:719–33.
Article18. Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006; 8:1074–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of O-Linked-N-Acetylglucosamine Modification and Its Associated Enzymes in Human Degenerated Intervertebral Discs
- Expression of Involucrin and Filaggrin in Various Skin Disorders: Immunohistochemical Study
- Immunogistochemical Studies with Carcinoembryonic Antigen in Various Tumors of Skin
- The Expression of beta-defensin 1 in Various Skin Tumors
- Regulatory Effects of O-GlcNAcylation in Vascular Smooth Muscle Cells on Diabetic Vasculopathy