Prog Med Phys.
2013 Sep;24(3):162-170. 10.14316/pmp.2013.24.3.162.
A Low-Dose High-Resolution SPECT System with CdTe for Small-Animal Imaging Applications: A GATE Simulation Study
- Affiliations
-
- 1Department of Radiological Science, College of Health Science, Research Institute of Health Science, Yonsei University, Wonju, Korea. hjk1@yonsei.ac.kr
- KMID: 1910580
- DOI: http://doi.org/10.14316/pmp.2013.24.3.162
Abstract
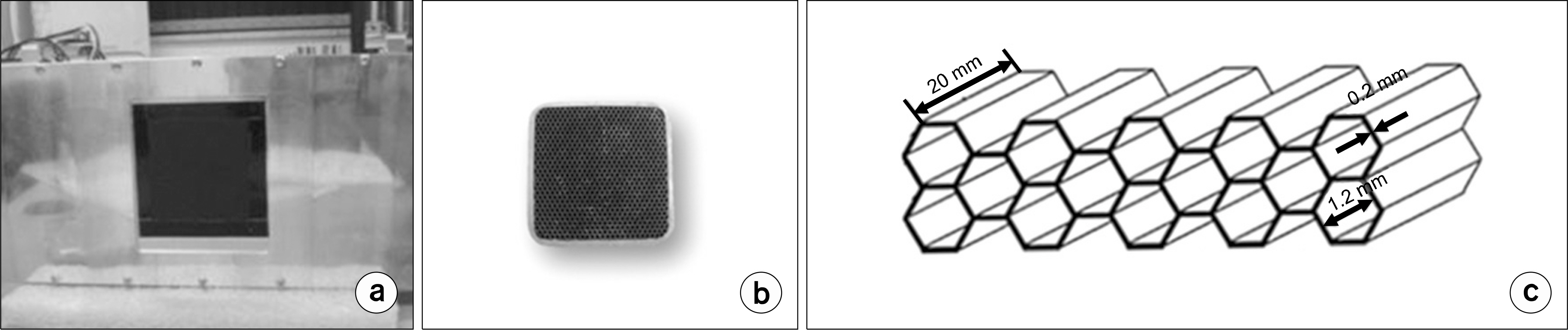
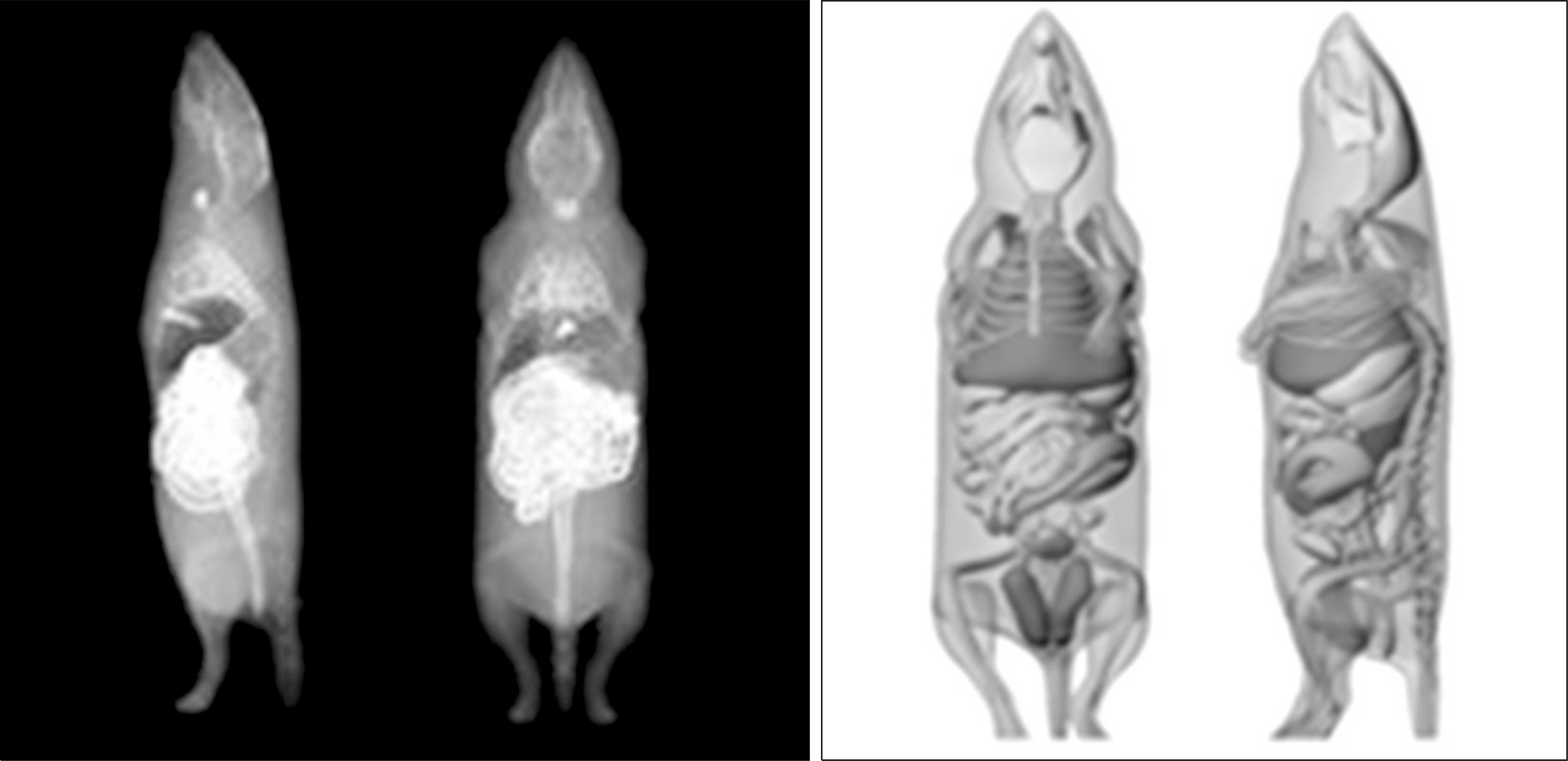
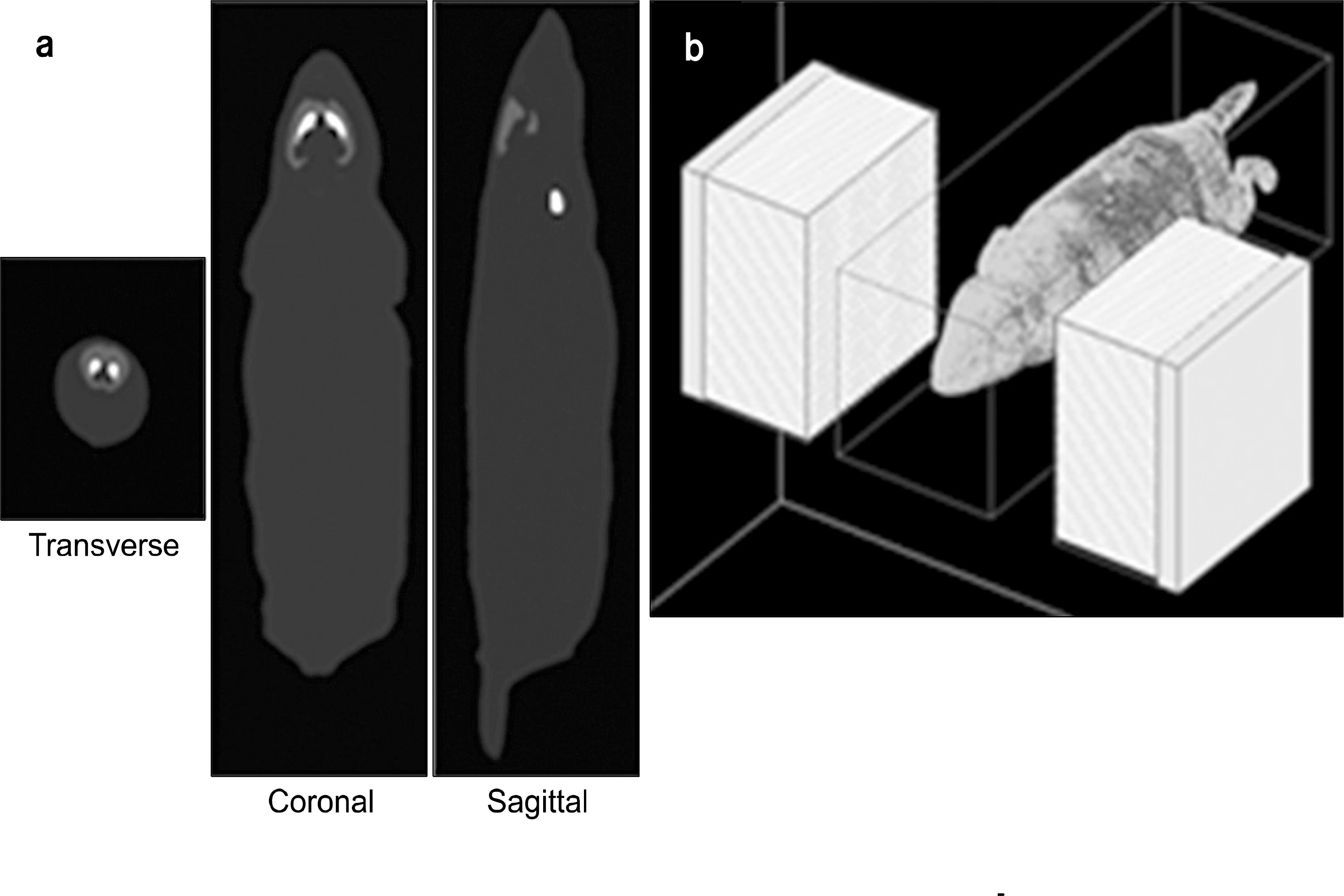
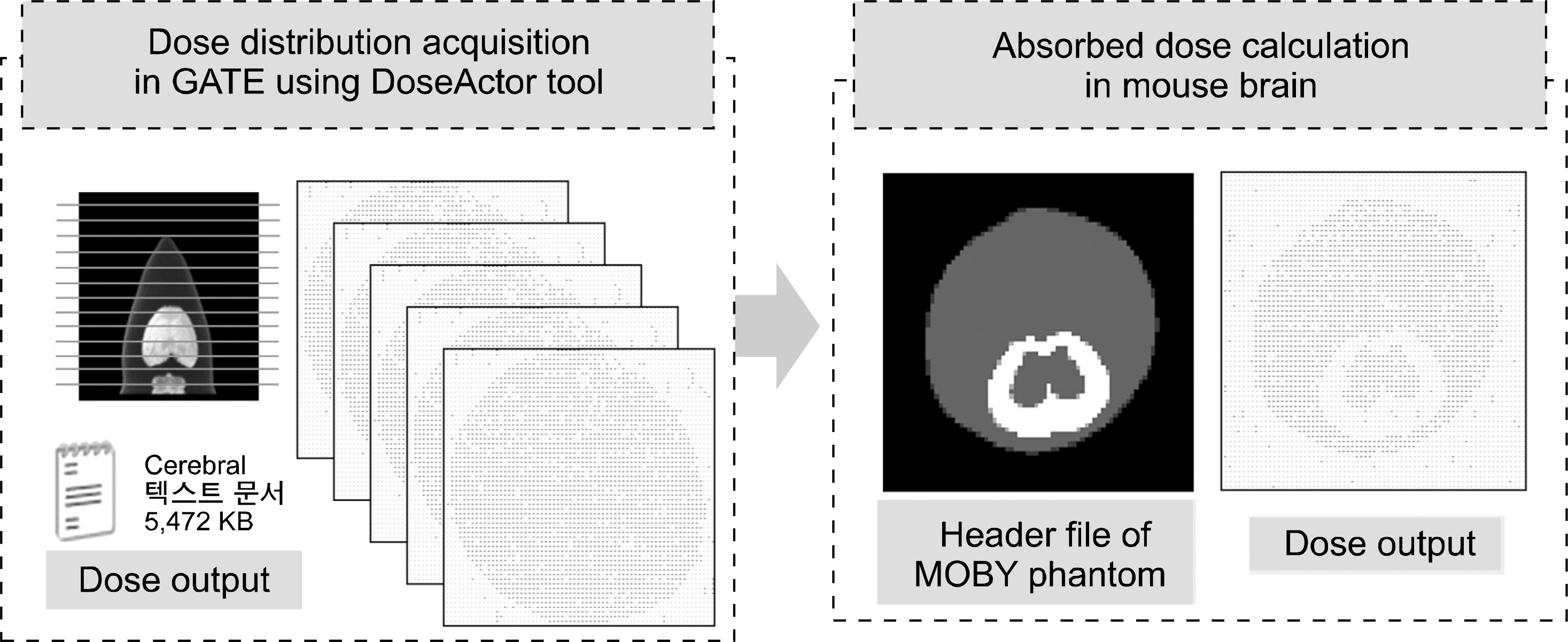
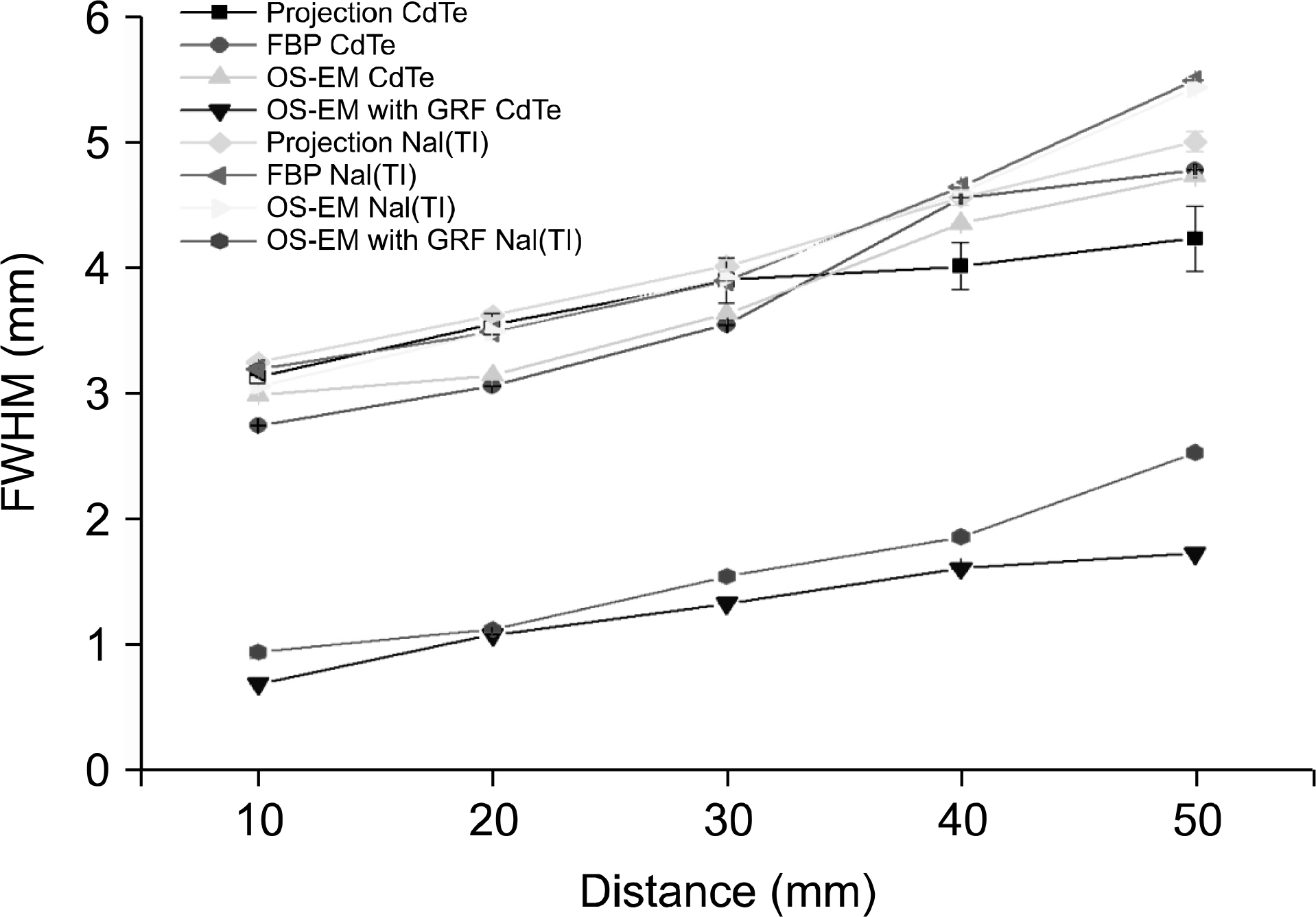
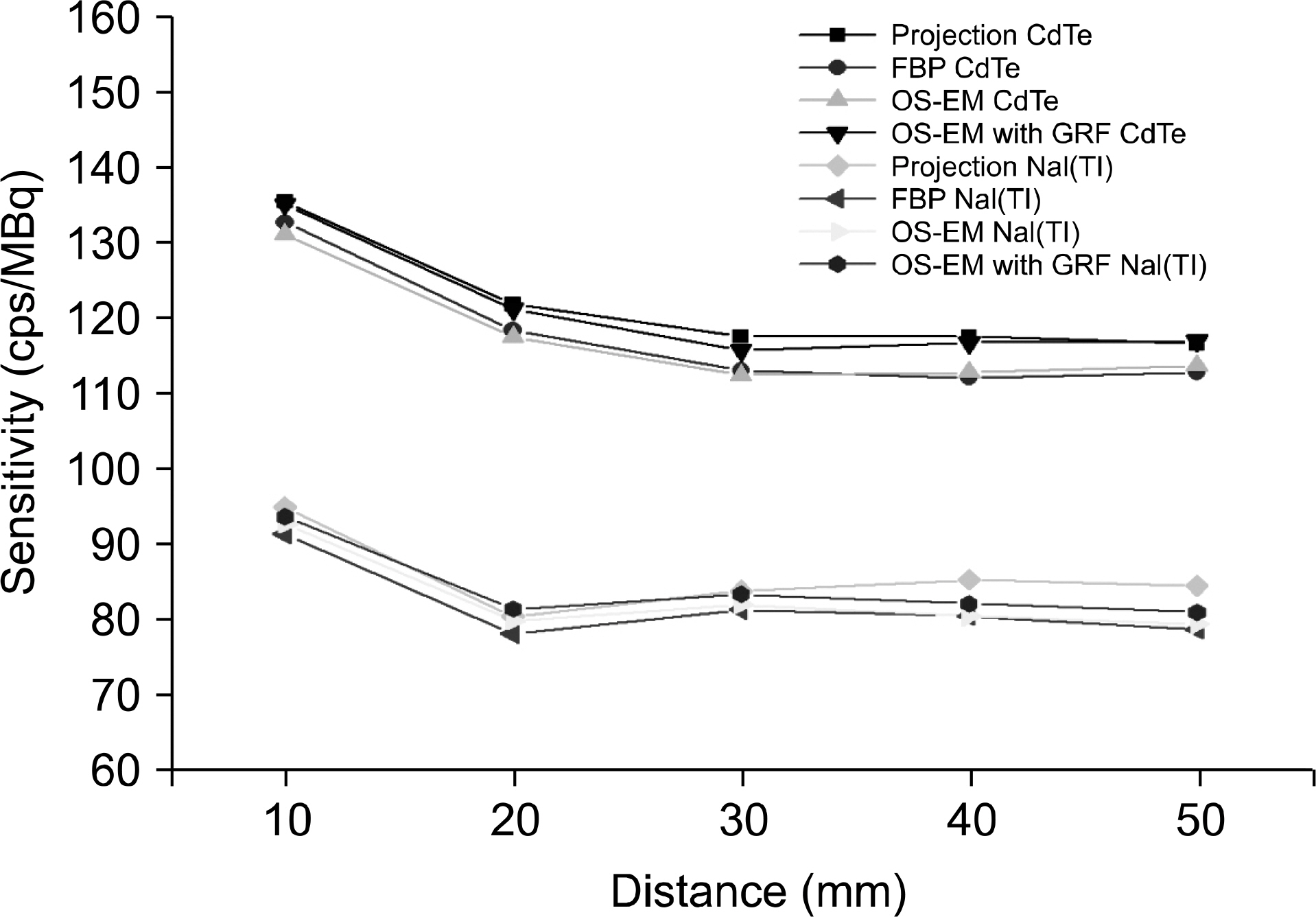
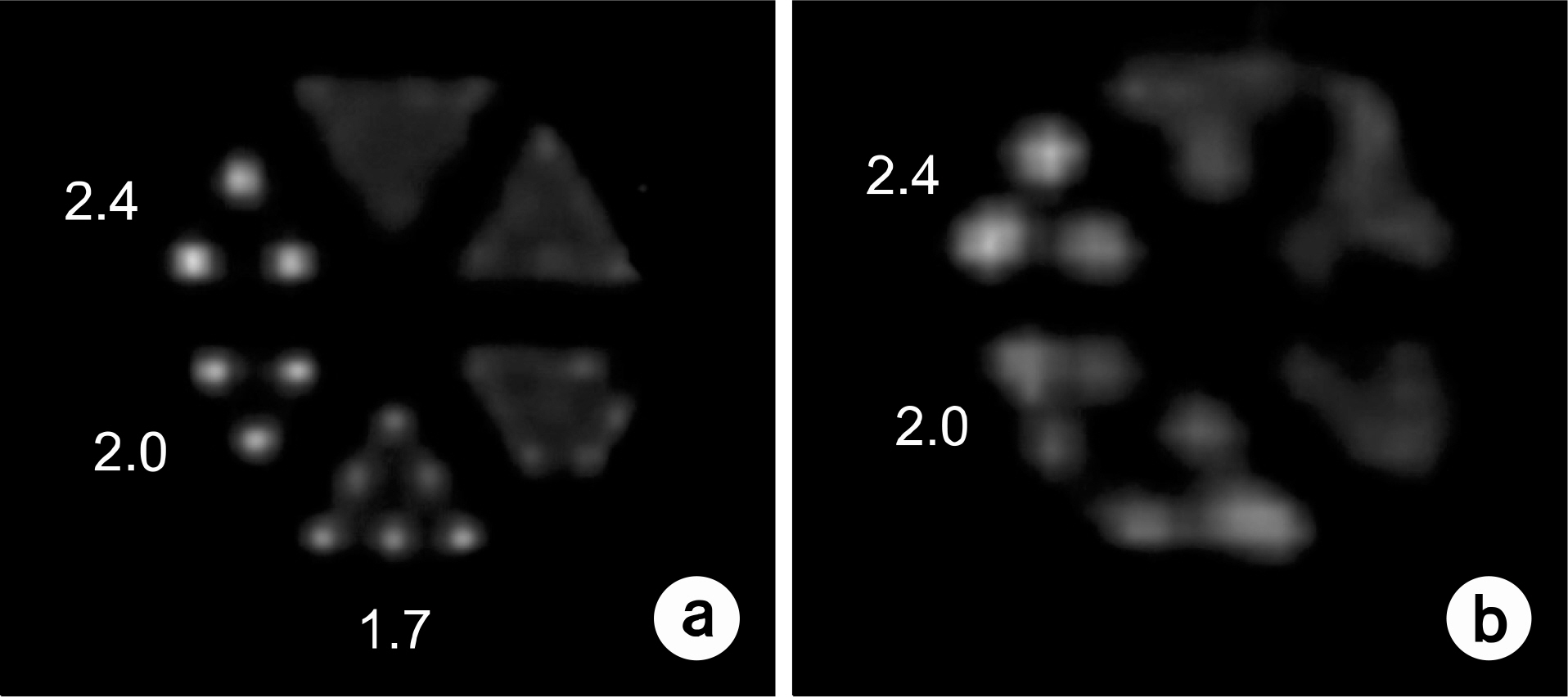
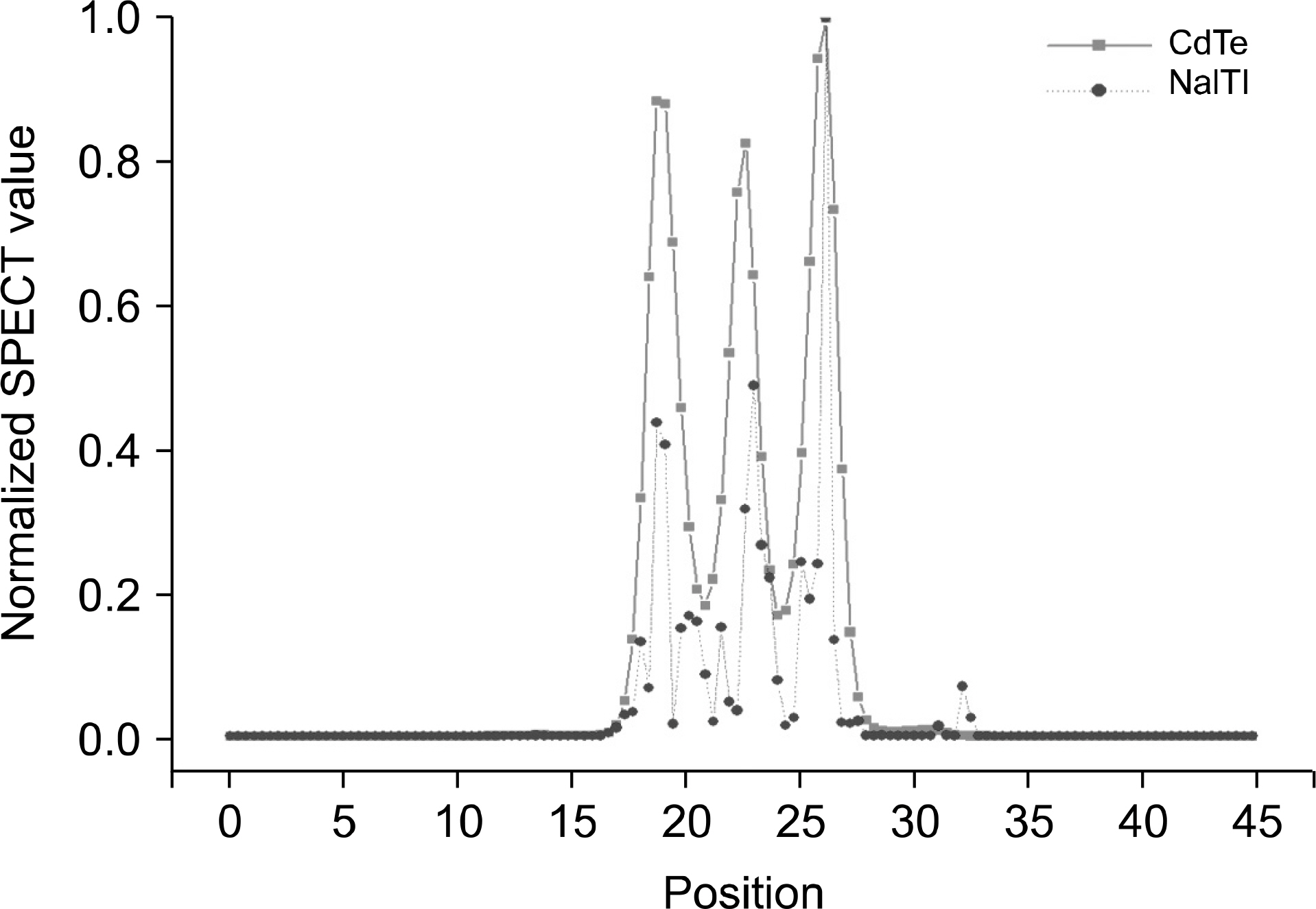
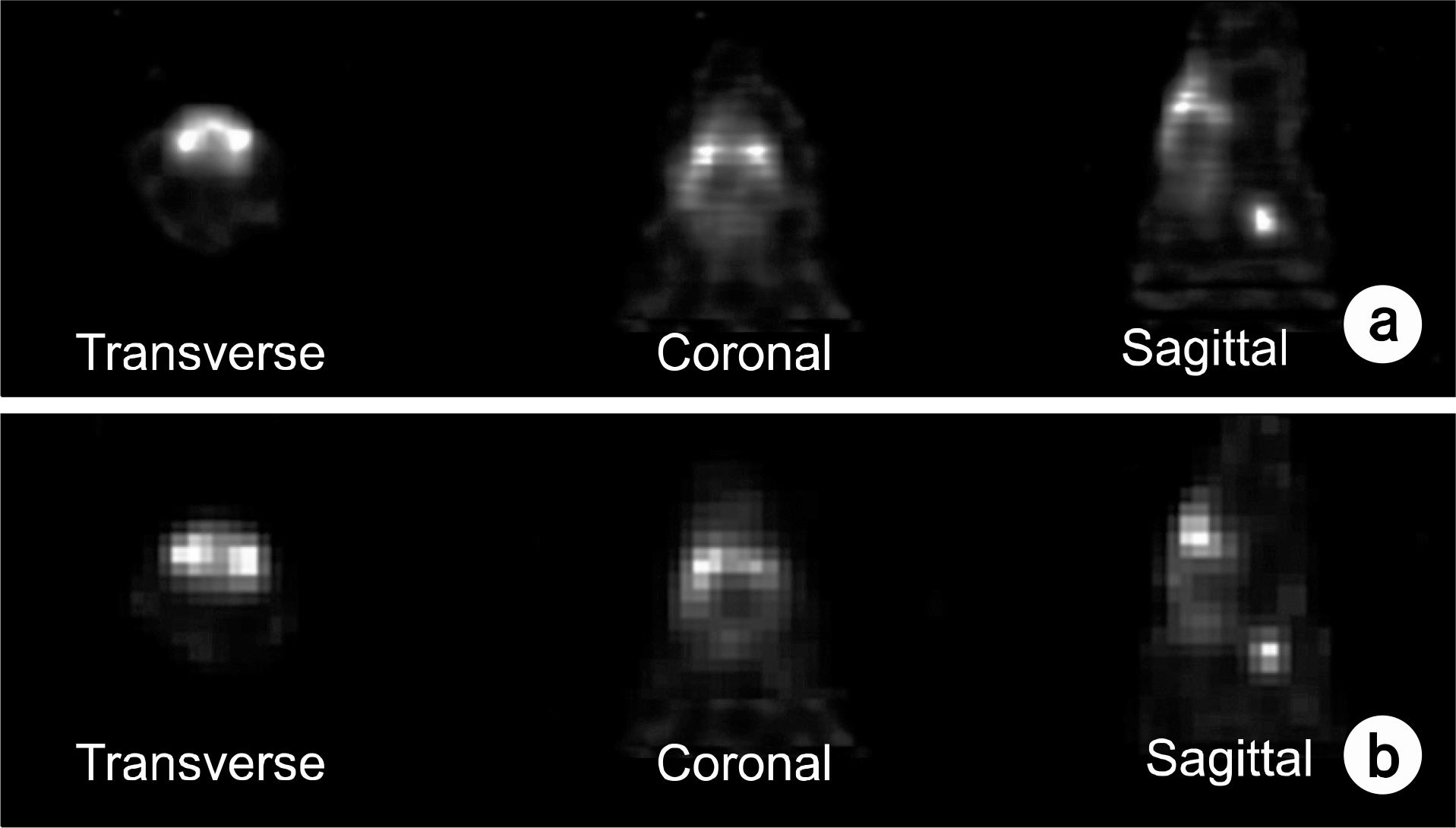
- Dedicated single-photon emission computed tomography (SPECT) systems based on pixelated semiconductors are being developed for studying small animal models of human disease. To clarify the possibility of using a SPECT system with CdTe for a high resolution low-dose small animal imaging, we compared the quality of reconstructed images from pixelated CdTe detector to those from a small SPECT system with NaI(Tl). The CdTe detector was 44.8x44.8 mm and the pixels were 0.35x0.35x5 mm. The intrinsic resolution of the detector was 0.35 mm, which is equal to the pixel size. GATE simulations were performed to assess the image quality of both SPECT systems. The spatial resolutions and sensitivities for both systems were evaluated using a 10 MBq 99mTc point source. The quantitative comparison with different injected dose was performed using a voxelized MOBY phantom, and the absorbed doses for each organ were evaluated. The spatial resolution of the SPECT with NaI(Tl) was about 1.54 mm FWHM, while that of the SPECT with a CdTe detector was about 1.32 mm FWHM at 30 mm. The sensitivity of NaI(Tl) based SPECT was 83 cps/MBq, while that of the CdTe detector based SPECT was 116 cps/MBq at 30 mm. The image statistics were evaluated by calculating the CNR of the image from both systems. When the injected activity for the striatum in the mouse brain was 160 Bq/voxel, the CNR of CdTe based SPECT was 2.30 while that of NaI(Tl) based SPECT was 1.85. The CNR of SPECT with CdTe was overall higher than that of the NaI(Tl) based SPECT. In addition, the absorbed dose was higher from SPECT with CdTe than those from NaI(Tl) based SPECT to acquire the same quantitative values. Our simulation results indicated that the SPECT with CdTe detector showed overall high performance compared to the SPECT with NaI(Tl). Even though the validation study is needed, the SPECT system with CdTe detector appeared to be feasible for high resolution low-dose small animal imaging.
Keyword
MeSH Terms
Figure
Reference
-
1. Meikle SR, Kench P, Kassiou M, Banati RB. Small animal SPECT and its place in the matrix of molecular imaging technologies. Phys Med Biol. 50(22):R45–R61. 2005.
Article2. Todd EP, Lasrs RF. SPECT detectors: the Anger Camera and beyond. Phys Med Biol. 56(17):R145–R182. 2011.3. Kim H, Furenlid LR, Crawford MJ, et al. SemiSPECT: a small-animal single-photon emission computed tomography (SPECT) imager based on eight cadmium zinc telluride (CZT) detector arrays. Med Phys. 33(2):465–474. 2006.
Article4. Park S, Lee C, Cho H, Kim H. Ultra-high-resolution SPECT with CdTe for small-animal imaging applications: a monte carlo simulation study using a voxelized phantom. JKPS. 60(7):1145–1149. 2012.
Article5. Kubo N, Songji A, Fujiki Y, et al. Evaluating performance of a pixel array semiconductor SPECT system for small animal imaging. Ann Nucl Med. 19(7):633–639. 2005.
Article6. Ogawa K, Muraishi M. Simulation Study on an ultra-high resolution SPECT with CdTe detectors. IEEE Nuclear Science Symposium Conference Record. 2006, San Diego, M10-50.7. Ogawa K, Ohmura N, Iida H, Kubo A. Development of an ultra-high resolution SPECT system with a CdTe semiconductor detector. Ann Nucl Med. 23(8):763–770. 2009.
Article8. Richard T, Arion FC. Monte Carlo simulations of absorbed dose in a mouse phantom from 18-fluorine compounds. Med Phys. 34(3):1026–1036. 2007.9. Papadimitroulas P, Nikiforidis GC, Kagadis GC. A dose point kernel database using GATE Monte Carlo simulation toolkit for nuclear medicine applications: Comparison with other Monte Carlo codes. Med Phys. 39(8):5238–5247. 2012.
Article10. Stolin AV, Williams MB, Kundu BK, et al. Characterization of imaging gamma detectors for use in small animal SPECT. Proc. IEEE Nuclear Science Symposium Conference Record. 2003, Portland, pp. 2085.
Article11. Jan S, Benoit D, Becheba E, Carlier T, et al. GATE V6: a major enhancement of the GATE simulation platform enabling modeling of CT and radiotherapy. Phys Med Biol. 56(4):881–901. 2011.12. Jan S, Santin G, Strul D, et al. GATE: a simulation toolkit for PET and SPECT. Phys Med Biol. 49(19):4543–4561. 2004.13. Assie K, Gardin I, Vera P, Buvat I. Validation of the Monte Carlo simulator GATE for indium-111 imaging. Phys Med Biol. 50(13):3113–3125. 2005.14. Staelens S, Strul D, Santin G, et al. Monte Carlo simulations of a scintillation camera using GATE: validation and application modeling. Phys Med Biol. 48(18):3021–3042. 2003.15. Zaidi H. Quantitative Analysis in Nuclear Medicine Imaging. Switzerland: Springer, Geneva;2006. ), pp.p. 141–165.16. Paul W, Tsui BMW. MCAT to XCAT: the evolution of 4-D computerized phantoms for imaging research. Proceedings of the IEEE. 2009, Orland, pp.1954–1968.17. Branco S, Jan S, Almeida P. Respiratory motion modeling in small animal PET using GATE. IEEE Nuclear Science Symposium Conference Record. 2008, Dresden, M10-234.18. Paul W, Tsui BMW, Frey FC, Johnson JGA, Berr SS. Development of a 4-D digital mouse phantom for molecular imaging research. Mol Imag Biol. 6(3):149–159. 2004.19. Cao Z, Bal G, Accorsi R, Acton PD. Optimal number of pinholes in multi-pinhole SPECT for mouse brain imaging-a simulation study. Phy Med Biol. 50(19):4609–4624. 2005.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Capabilities of theMonte Carlo Simulation Codes forModeling of a Small Animal SPECT Camera
- Development of a High Resolution SPECT Detector with Depth-encoding Capability for Multi-energy Imaging: Monte Carlo Simulation
- A Computer Simulation for Small Animal Iodine-125 SPECT Development
- Coded Aperture Gamma Camera for Thyroid Imaging: Monte Carlo Simulation
- Development of GATE Monte Carlo Code for Simulation and Dosimetry of New I-125 Seeds in Eye Plaque Brachytherapy