J Korean Soc Spine Surg.
2013 Dec;20(4):210-214. 10.4184/jkss.2013.20.4.210.
Anatomical and Pathophysiological Features of Cauda Equina
- Affiliations
-
- 1Department of Orthopedic Surgery, Chonbuk National Univeristy School of Medicine, Biomedical Research Institute, Chonbuk National University Hospital, Jeonju, Korea. osdr2815@naver.com
- KMID: 1896953
- DOI: http://doi.org/10.4184/jkss.2013.20.4.210
Abstract
- STUDY DESIGN: Review of literature on anatomical and pathophysiological features of cauda equina.
OBJECTIVES
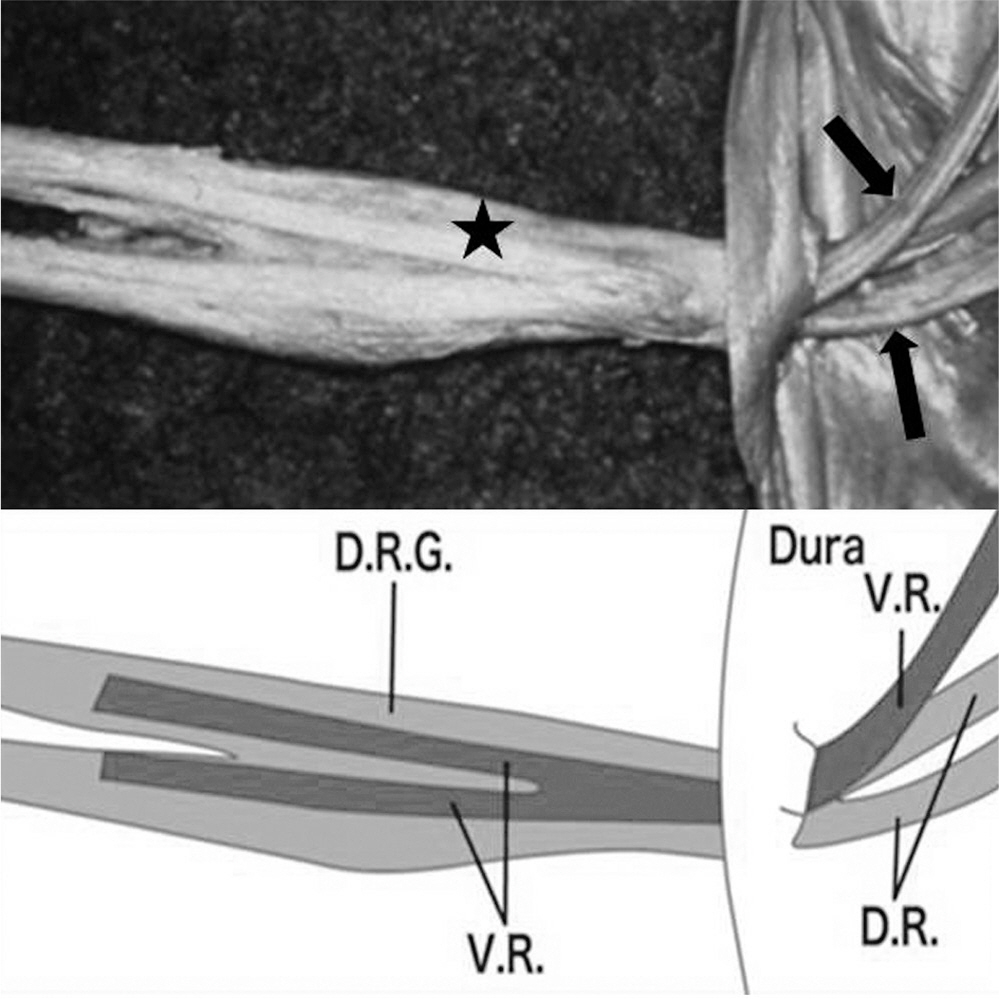
To look into the anatomical and pathophysiological features of cauda equina and support their basic knowledge of treating cauda equina syndrome. SUMMARY OF LITERATURE REVIEW: Cauda equina has different anatomical and pathophysiological features to peripheral nerve.
MATERIALS AND METHODS
Review of literature.
RESULTS
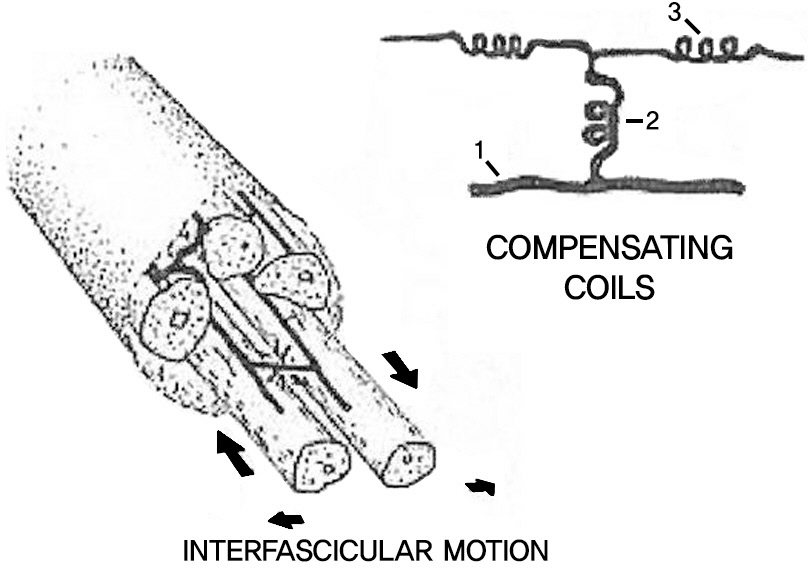
When compressing to cauda equina, the pathophysiologic mechanism develop as follows; increasing the vascular permiablity of nerve root, intraneural edema, and subsequent blood and nutritional impairment. Nerve root injury develops through this pathophysiologic mechanism.
CONCLUSIONS
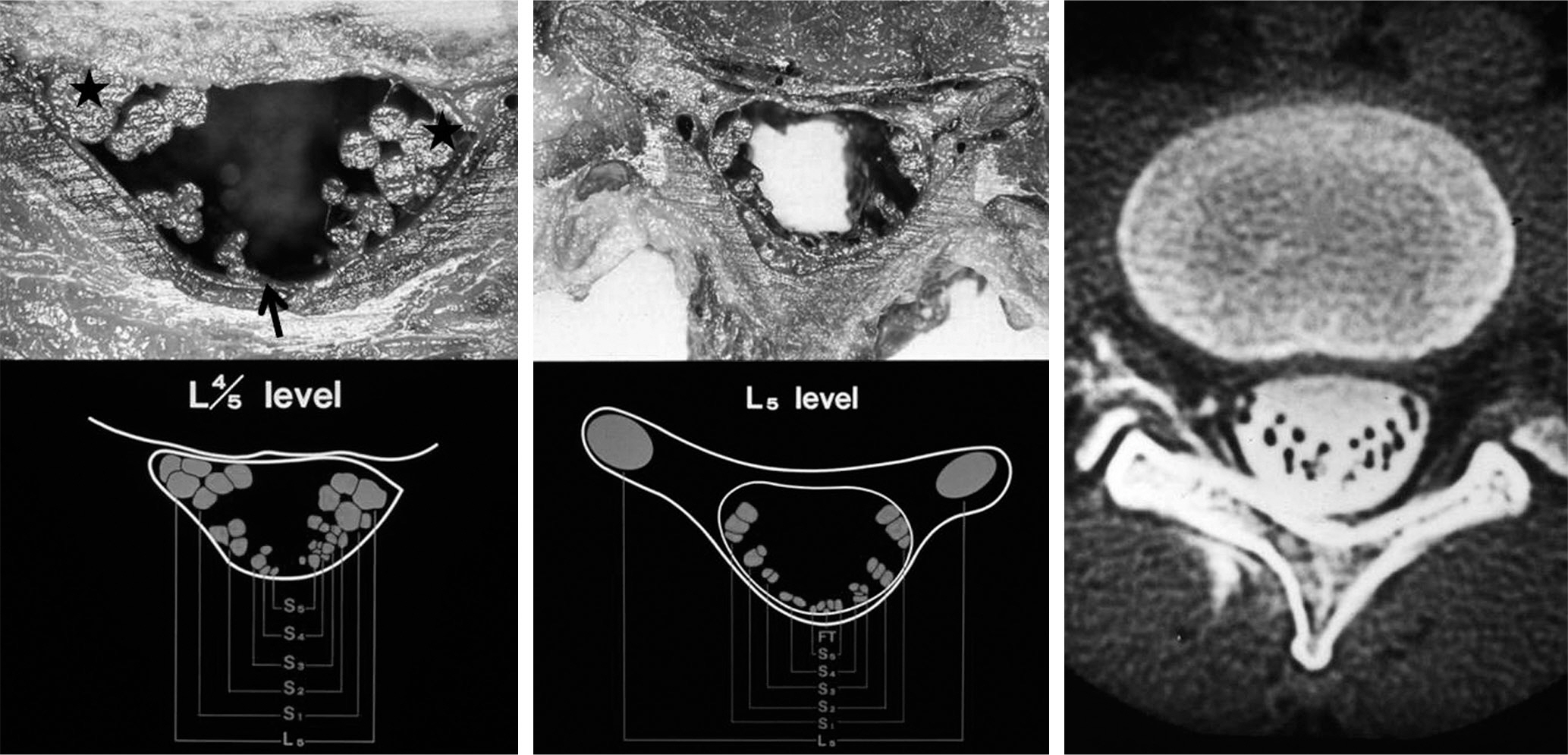
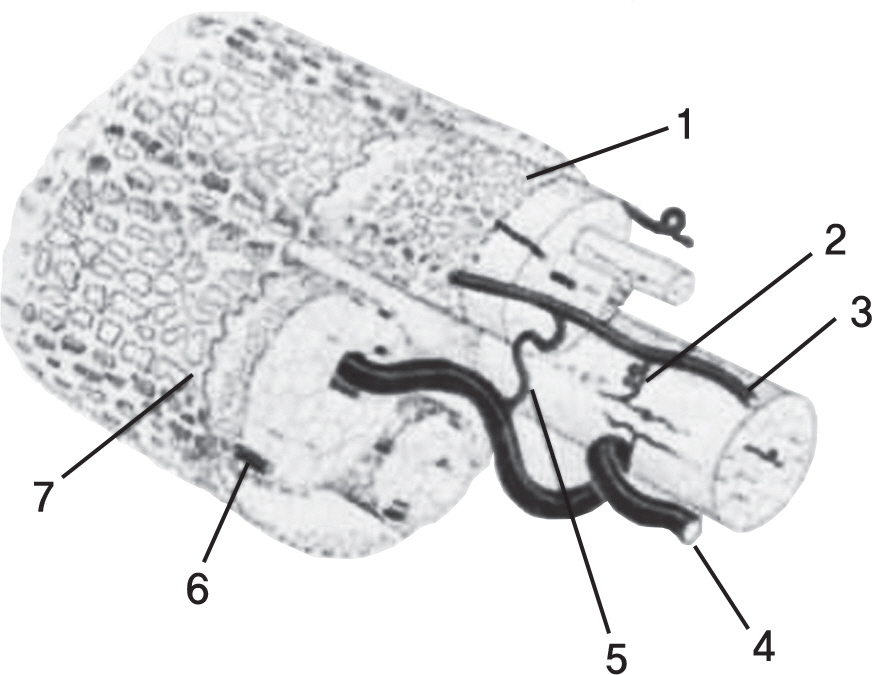
Cauda equina has an extensive ateriovenous anastomosis and guaze-like pia mater, which supply blood and neutrition to it. These anatomical features prevent it from complete cauda equina syndrome when compressing to it under arterial blood pressure.
Keyword
Figure
Reference
-
1. Konno S, Olmarker K, Byrod G, Rydevik B, Kikuchi S. Intermittent cauda equina compression: an experimental study of the porcine cauda equina with analyses of nerve impulse conduction properties. Spine (Phila Pa 1976). 1995; 20:1223–6.
Article2. Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S. A model for acute, chronic and delayed graded compression of the dog cauda equina. Presentation of the gross, microscopic and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine (Phila Pa 1976). 1996; 20:2758–64.3. Olmarker K, Rydevik B. Single versus double level nerve root compression: an experimental study on the porcine cauda equina with analyses of nerve impulse conduction properties. Clin Orthop. 1992; 279:35–9.4. Olmarker K, Rydevik B, Hansson T, Holm S. Compression-induced changes of the nutritional supply to the porcine cauda equina. J Spinal Disord. 1990; 3:25–9.
Article5. Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine (Phila Pa 1976). 1989; 14:569–73.6. Olmarker K, Rydevik B, Holm S, Bagge U. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J Orthop Res. 1989; 7:817–23.
Article7. Olmarker K, Takahashi K, Rydevik B. Anatomy and compression-pathophysiology of the nerve roots of the lumbar spine. Anderson G.B.J., MacNeill T., editors(Eds.),. Spinal Stenosis. St. Louis: Mosby Year Book;1992. p. 77–90.8. Orendacova J, Cızkova D, Kafka J, et al. Cauda equina syndrome. Progress in Neurobiology. 2011. 613–37.
Article9. Yonetake T, Sekiguchi M, Konno S, Kikuchi S, Kanaya F. Compensatory Neovascularization After Cauda Equina Compression in Rats. Spine (Phila Pa 1976). 2008; 33:140–5.
Article10. Pedowitz RA, Garfin SR, Massie JB, et al. Effects of magnitude and duration of compression on spinal nerve root conduction. Spine (Phila Pa 1976). 1992; 17:194–9.
Article11. Takahashi K, Olmarker K, Holm S, Porter RW, Rydevik B. Double-level cauda equina compression: an experimental study with continuous monitoring of intraneural blood flow in the porcine cauda equina. J. Orthop. Res. 1993; 11:104–9.12. Parke WW, Gammel K, Rothman RH. Arterial vascularization of the cauda equine. J Bone Joint Surg Am. 1981; 63:53–62.13. Parke WW, Watanabe R. The intrinsic vasculature of the lumbosacral spinal nerve roots. Spine (Phila Pa 1976). 1985; 10:508–15.