Lab Anim Res.
2011 Sep;27(3):205-212. 10.5625/lar.2011.27.3.205.
Chemical compound 31002 stimulates cardiomyogenic differentiation of embryonic stem cells
- Affiliations
-
- 1Biomedical Mouse Resource Center, KRIBB, Ochang-eup, Korea. namk@kribb.re.kr
- 2Center for Regenerative Medicine, KRIBB, Daejeon, Korea.
- 3Department of Chemistry, Kangwon National University, Chuncheon, Korea.
- KMID: 1850047
- DOI: http://doi.org/10.5625/lar.2011.27.3.205
Abstract
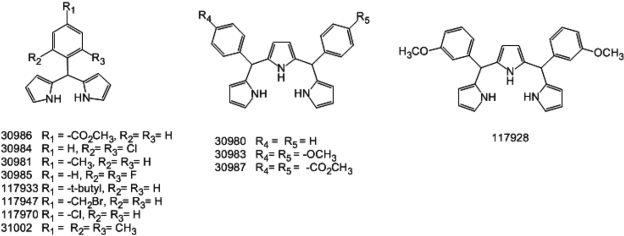
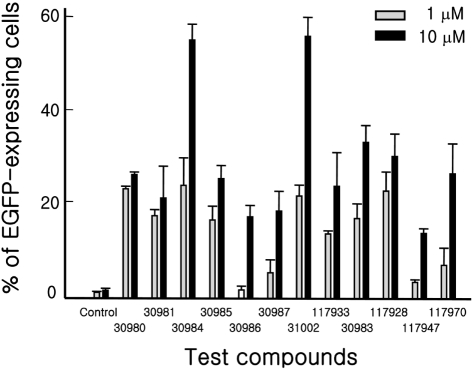
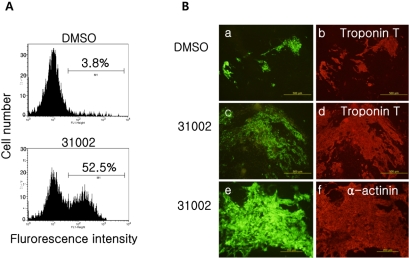
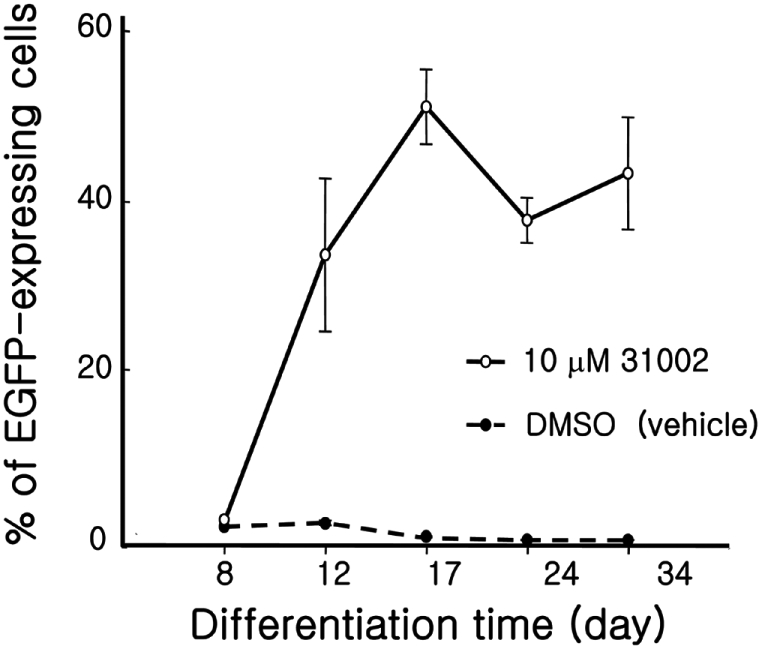
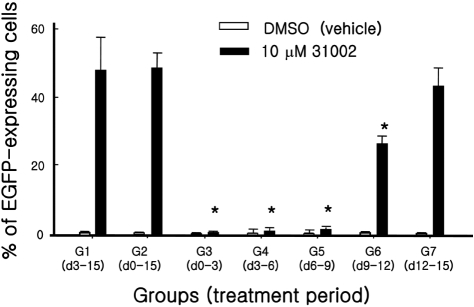
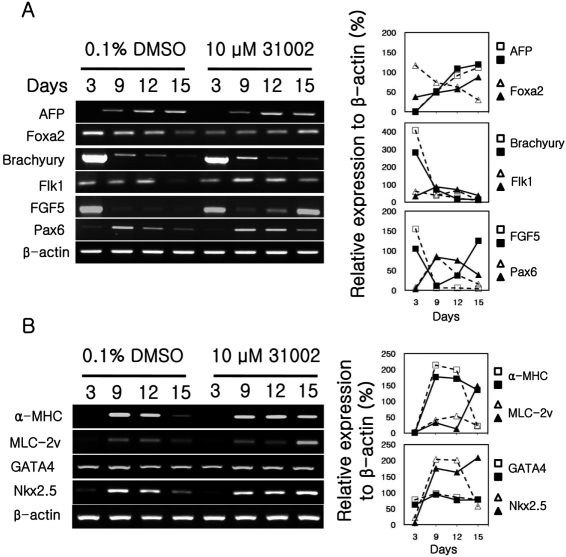
- Embryonic stem cells (ESCs) are an emerging source for cell-based therapies aimed at repairing damaged organ tissues; however, the efficiency of directed differentiation is low and refinement of differentiation protocols is hampered by incomplete understanding of the mechanisms involved in this process. To find new compounds which can improve the efficiency of directed differentiation of ESCs to cardiomyocytes, we screened several thousand chemical compounds and identified a promising group. All of the compounds found have a common structure of 1H-pyrrole,2,2'-(phenylmethylene)bis. Here we report the potential mechanism of action for 31002 which showed the strongest activity among the compounds selected. In the presence of 31002, 15 times more cardiomyocytes differentiated from ESCs, i.e., 3.5% to 52% of total differentiated cells. Moreover, the cardiomyocytes showed functional characteristics including rhythmic beating and marker gene expression. 31002 inhibited the down-regulation of genes related to the three germ layers in the late stage of ESCs differentiation, implying that 31002 supports a continuous fate commitment of undifferentiated ESCs to the cardiac lineage by prolonging the three germ layer stages. Therefore, compounds in this group, including 31002, might be useful as directed cardiomyogenic differentiation-inducers to produce cells for use in cell therapy aimed at restoring damaged heart tissue.
Keyword
MeSH Terms
Figure
Reference
-
1. Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, Mockrin SC, Reinlib L. Report of the National Heart, Lung, and Blood Institute Special Emphasis Panel on Heart Failure Research. Circulation. 1997; 95(4):766–770. PMID: 9054723.
Article2. Puceat M. Human embryonic stem cell: is it a realistic cell source for regenerative therapy of heart failure? Pathol Biol (Paris). 2008; 56(2):47–49. PMID: 18178334.3. Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002; 90(10):1044–1054. PMID: 12039793.
Article4. Kusumoto FM, Goldschlager N. Cardiac pacing. N Engl J Med. 1996; 334(2):89–97. PMID: 8531965.
Article5. Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001; 108(3):407–414. PMID: 11489934.6. Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006; 8(1):105–110. PMID: 16387630.
Article7. Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci USA. 2003; 100(18):10440–10445. PMID: 12928492.
Article8. Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003; 114(6):763–776. PMID: 14505575.
Article9. Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005; 433(7026):647–653. PMID: 15703750.10. Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007; 13(8):970–974. PMID: 17660827.
Article11. Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007; 13(8):962–969. PMID: 17632525.
Article12. Ménard C, Hagège AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, Bel A, Messas E, Bissery A, Bruneval P, Desnos M, Pucéat M, Menasché P. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005; 366(9490):1005–1012. PMID: 16168783.
Article13. Tomescot A, Leschik J, Bellamy V, Dubois G, Messas E, Bruneval P, Desnos M, Hagège AA, Amit M, Itskovitz J, Menasché P, Pucéat M. Differentiation in vivo of cardiac committed human embryonic stem cells in postmyocardial infarcted rats. Stem Cells. 2007; 25(9):2200–2205. PMID: 17540853.14. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282(5391):1145–1147. PMID: 9804556.
Article15. Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, Ji G, Fleischmann B, Katus HA, Hescheler J, Franz WM. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol. 1997; 29(6):1525–1539. PMID: 9220339.
Article16. Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003; 107(14):1912–1916. PMID: 12668514.
Article17. Paquin J, Danalache BA, Jankowski M, McCann SM, Gutkowska J. Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Natl Acad Sci USA. 2002; 99(14):9550–9555. PMID: 12093924.
Article18. Kim EK, Seo YJ, Oh GT, Yoon M, Lee Y, Kim HC, Choi YK, Lee CH, Kang JS, Nam KH. Fluorescence labeled cardiomyocytes derived from embryonic stem cells. Lab Anim Res. 2005; 21(1):60–68.19. Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002; 91(3):189–201. PMID: 12169644.
Article20. Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005; 23(5):607–611. PMID: 15867910.
Article21. Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006; 13(9):957–963. PMID: 16984885.
Article22. Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002; 16(12):1558–1566. PMID: 12374778.
Article23. Min JY, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, Morgan JP, Xiao YF. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003; 125(2):361–369. PMID: 12579106.
Article24. Kanno S, Kim PK, Sallam K, Lei J, Billiar TR, Shears LL 2nd. Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2004; 101(33):12277–12281. PMID: 15304656.
Article25. Sauer H, Rahimi G, Hescheler J, Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000; 476(3):218–223. PMID: 10913617.
Article26. Sato H, Takahashi M, Ise H, Yamada A, Hirose S, Tagawa Y, Morimoto H, Izawa A, Ikeda U. Collagen synthesis is required for ascorbic acid-enhanced differentiation of mouse embryonic stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006; 342(1):107–112. PMID: 16480687.
Article27. Menard C, Grey C, Mery A, Zeineddine D, Aimond F, Puceat M. Cardiac specification of embryonic stem cells. J Cell Biochem. 2004; 93(4):681–687. PMID: 15389971.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nuclear receptor regulation of stemness and stem cell differentiation
- Enhanced Cardiomyogenic Differentiation of P19 Embryonal Carcinoma Stem Cells
- Current Concepts of Stem Cell Therapy
- Assessment of Developmental Toxicants using Human Embryonic Stem Cells
- Pja2 Inhibits Wnt/β-catenin Signaling by Reducing the Level of TCF/LEF1