Tuberc Respir Dis.
2014 May;76(5):211-217. 10.4046/trd.2014.76.5.211.
Malignant Pleural Effusion: Medical Approaches for Diagnosis and Management
- Affiliations
-
- 1Center for Lung Cancer and Division of Pulmonary, Department of Internal Medicine, Inha University Hospital, Inha University College of Medicine, Incheon, Korea. hsnam@inha.ac.kr
- KMID: 1842966
- DOI: http://doi.org/10.4046/trd.2014.76.5.211
Abstract
- Malignant pleural effusions (MPEs) are the second leading cause of exudative pleural effusions after parapneumonic effusions. In the vast majority of cases, a MPE signifies incurable disease associated with high morbidity and mortality. Considerable advances have been made for the diagnosis of MPEs, through the development of improved methods in the specialized cytological and imaging studies. The cytological or histological confirmation of malignant cells is currently important in establishing a diagnosis. Furthermore, despite major advancements in cancer treatment for the past two decades, management of MPE remains palliative. This article presents a comprehensive review of the medical approaches for diagnosis and management of MPE.
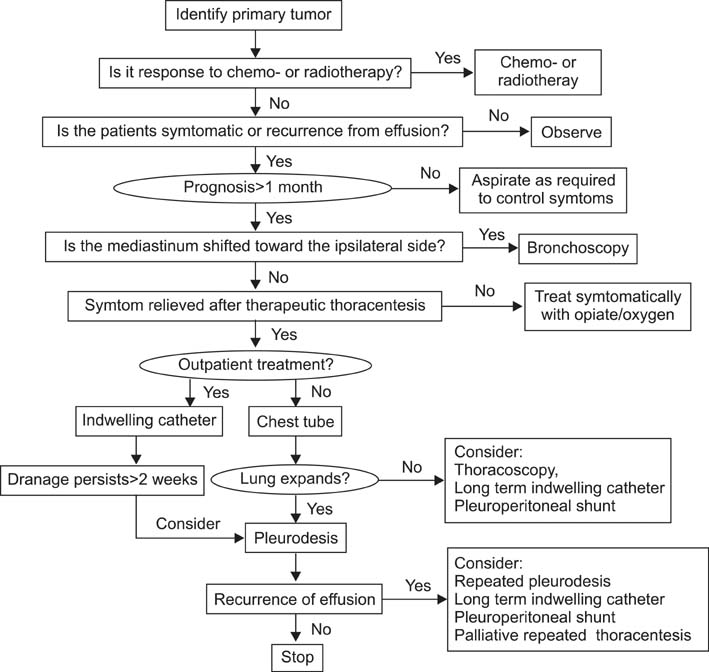
Figure
Reference
-
1. Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med. 2013; 34:459–471.2. Light RW. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins;2013.3. Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008; 83:235–250.4. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest. 2000; 117:79–86.5. Bielsa S, Salud A, Martinez M, Esquerda A, Martin A, Rodriguez-Panadero F, et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med. 2008; 19:334–339.6. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007; 2:706–714.7. Ryu JS, Ryu HJ, Lee SN, Memon A, Lee SK, Nam HS, et al. Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol. 2014; 32:960–967.8. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977; 63:695–702.9. Alkhawaldeh K, Biersack HJ, Henke A, Ezziddin S. Impact of dual-time-point F-18 FDG PET/CT in the assessment of pleural effusion in patients with non-small-cell lung cancer. Clin Nucl Med. 2011; 36:423–428.10. Ashchi M, Golish J, Eng P, O'Donovan P. Transudative malignant pleural effusions: prevalence and mechanisms. South Med J. 1998; 91:23–26.11. Ryu JS, Ryu ST, Kim YS, Cho JH, Lee HL. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003; 18:230–233.12. Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang J, Yang HB. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax. 2008; 63:35–41.13. Shi HZ, Liang QL, Jiang J, Qin XJ, Yang HB. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology. 2008; 13:518–527.14. Ishimoto O, Saijo Y, Narumi K, Kimura Y, Ebina M, Matsubara N, et al. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology. 2002; 63:70–75.15. Shen YC, Liu MQ, Wan C, Chen L, Wang T, Wen FQ. Diagnostic accuracy of vascular endothelial growth factor for malignant pleural effusion: a meta-analysis. Exp Ther Med. 2012; 3:1072–1076.16. Davies HE, Sadler RS, Bielsa S, Maskell NA, Rahman NM, Davies RJ, et al. Clinical impact and reliability of pleural fluid mesothelin in undiagnosed pleural effusions. Am J Respir Crit Care Med. 2009; 180:437–444.17. Pass HI, Levin SM, Harbut MR, Melamed J, Chiriboga L, Donington J, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med. 2012; 367:1417–1427.18. Porcel JM. Pleural fluid biomarkers: beyond the Light criteria. Clin Chest Med. 2013; 34:27–37.19. Sriram KB, Relan V, Clarke BE, Duhig EE, Yang IA, Bowman RV, et al. Diagnostic molecular biomarkers for malignant pleural effusions. Future Oncol. 2011; 7:737–752.20. Loddenkemper R. Thoracoscopy: state of the art. Eur Respir J. 1998; 11:213–221.21. Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003; 361:1326–1330.22. Musani AI. Treatment options for malignant pleural effusion. Curr Opin Pulm Med. 2009; 15:380–387.23. Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010; 65:Suppl 2. ii32–ii40.24. Uzbeck MH, Almeida FA, Sarkiss MG, Morice RC, Jimenez CA, Eapen GA, et al. Management of malignant pleural effusions. Adv Ther. 2010; 27:334–347.25. Myers R, Michaud G. Tunneled pleural catheters: an update for 2013. Clin Chest Med. 2013; 34:73–80.26. Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012; 307:2383–2389.27. Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration. 2012; 83:91–98.28. Tan C, Sedrakyan A, Browne J, Swift S, Treasure T. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006; 29:829–838.29. Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med. 1994; 120:56–64.30. Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev. 2004; (1):CD002916.31. Paschoalini Mda S, Vargas FS, Marchi E, Pereira JR, Jatene FB, Antonangelo L, et al. Prospective randomized trial of silver nitrate vs talc slurry in pleurodesis for symptomatic malignant pleural effusions. Chest. 2005; 128:684–689.32. Mohsen TA, Zeid AA, Meshref M, Tawfeek N, Redmond K, Ananiadou OG, et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg. 2011; 40:282–286.33. Ren S, Terman DS, Bohach G, Silvers A, Hansen C, Colt H, et al. Intrapleural staphylococcal superantigen induces resolution of malignant pleural effusions and a survival benefit in non-small cell lung cancer. Chest. 2004; 126:1529–1539.34. Janssen JP, Collier G, Astoul P, Tassi GF, Noppen M, Rodriguez-Panadero F, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007; 369:1535–1539.35. Puri V, Pyrdeck TL, Crabtree TD, Kreisel D, Krupnick AS, Colditz GA, et al. Treatment of malignant pleural effusion: a cost-effectiveness analysis. Ann Thorac Surg. 2012; 94:374–379.36. Nam HS, Ryu JS. Diagnosis and management of malignant pleural effusion. Korean J Med. 2011; 81:167–173.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Tools of Pleural Effusion
- The Utility of Pleural Adenosine Deaminase for Diagnosis of Differentiating Tuberculous Pleural Effusion in Children
- Diagnostic Value of LDH and its Isoenzyme in Pleural Effusion
- Four Cases of Malignant Pleural Effusion in Patients with Papillary Thyroid Carcinoma
- Diagnostic Value of Adenosine Deaminase(ADA) and its Isoenzyme in Pleural Effusion