J Korean Soc Radiol.
2014 Aug;71(2):89-96. 10.3348/jksr.2014.71.2.89.
Response Evaluation to Neoadjuvant Chemotherapy in Advanced Breast Cancer: Comparison of MRI and Positron Emission Tomography/CT (RECIST 1.1 versus PERCIST 1.0)
- Affiliations
-
- 1Department of Radiology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea. didi97@catholic.ac.kr
- 2Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1819786
- DOI: http://doi.org/10.3348/jksr.2014.71.2.89
Abstract
- PURPOSE
The aim of this study was to compare the diagnostic performances between the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) criteria on dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) and positron emission tomography Response Evaluation Criteria in Solid Tumors version 1.0 (PERCIST 1.0) criteria on positron emission tomography/CT (PET/CT) in evaluating the treatment response of neoadjuvant chemotherapy (NAC) in breast cancer patients.
MATERIALS AND METHODS
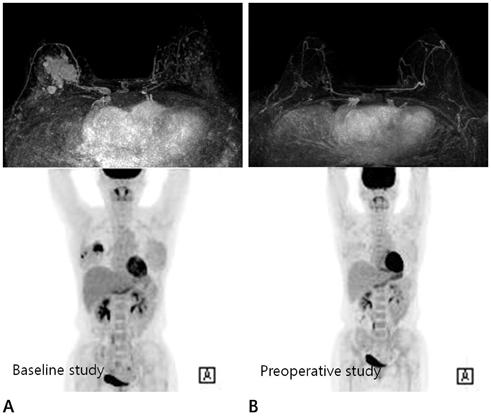
We compared MRI and PET/CT in the assessment of tumor response after NAC with the pathological response as the standard reference in 54 breast cancer patients. The tumor response was assessed by using the RECIST 1.1 criteria on DCE-MRI and PERCIST 1.0 criteria on PET/CT. The diagnostic performance of RECIST 1.1 and PERCIST 1.0 criteria was statistically analyzed and compared by receiver operating characteristic (ROC) analysis.
RESULTS
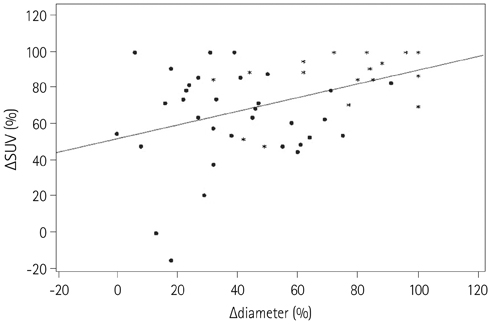
There were 21 responders and 33 non-responders according to the pathology. The discordant rate with pathological response was 37.0% for RECIST 1.1 and 55.6% for PERCIST 1.0. Twenty six patients (48.1%) were classified as responders in both MRI and PET/CT, but the final pathology showed non-response. In the ROC curve, the area under the curves (AUC) was 0.89 for RECIST 1.1 and 0.79 for PERCIST 1.0 (p < 0.001).
CONCLUSION
The RECIST 1.1 criteria on MRI showed the better diagnostic performance than PERCIST 1.0 criteria on PET/CT for the response assessment of breast cancer after NAC, although there was no statistically significant difference between both (p = 0.15).
MeSH Terms
Figure
Reference
-
1. Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008; 26:786–790.2. Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002; 95:681–695.3. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–785.4. Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002; 179:1193–1199.5. Londero V, Bazzocchi M, Del Frate C, Puglisi F, Di Loreto C, Francescutti G, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004; 14:1371–1379.6. Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005; 184:868–877.7. Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006; 42:1031–1039.8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.9. Kang H, Lee HY, Lee KS, Kim JH. Imaging-based tumor treatment response evaluation: review of conventional, new, and emerging concepts. Korean J Radiol. 2012; 13:371–390.10. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009; 50:Suppl 1. 122S–150S.11. Chen X, Moore MO, Lehman CD, Mankoff DA, Lawton TJ, Peacock S, et al. Combined use of MRI and PET to monitor response and assess residual disease for locally advanced breast cancer treated with neoadjuvant chemotherapy. Acad Radiol. 2004; 11:1115–1124.12. Choi JH, Lim HI, Lee SK, Kim WW, Kim SM, Cho E, et al. The role of PET CT to evaluate the response to neoadjuvant chemotherapy in advanced breast cancer: comparison with ultrasonography and magnetic resonance imaging. J Surg Oncol. 2010; 102:392–397.13. Dose-Schwarz J, Tiling R, Avril-Sassen S, Mahner S, Lebeau A, Weber C, et al. Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br J Cancer. 2010; 102:35–41.14. Park JS, Moon WK, Lyou CY, Cho N, Kang KW, Chung JK. The assessment of breast cancer response to neoadjuvant chemotherapy: comparison of magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography. Acta Radiol. 2011; 52:21–28.15. Tateishi U, Miyake M, Nagaoka T, Terauchi T, Kubota K, Kinoshita T, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging--prospective assessment. Radiology. 2012; 263:53–63.16. Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998; 16:93–100.17. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the morphologic criteria (RECIST) and metabolic criteria (EORTC and PERCIST) in tumor response assessments: a pooled analysis
- PERCIST in Perspective
- RECIST Criteria for Tumor Response in the Patients with Breast Cancer Who Had Neoadjuvant Chemotherapy
- Comparison of the Diagnostic Performance of Response Evaluation Criteria in Solid Tumor 1.0 with Response Evaluation Criteria in Solid Tumor 1.1 on MRI in Advanced Breast Cancer Response Evaluation to Neoadjuvant Chemotherapy
- Evaluation of RECIST, PERCIST, EORTC, and MDA Criteria for Assessing Treatment Response with Ga68-PSMA PET-CT in Metastatic Prostate Cancer Patient with Biochemical Progression: a Comparative Study