J Clin Neurol.
2005 Oct;1(2):134-141. 10.3988/jcn.2005.1.2.134.
Safety and Feasibility of Subcutaneous Low Molecular Weight Heparin for Cerebral Venous Sinus Thrombosis
- Affiliations
-
- 1Department of Neurology, College of Medicine, Chungnam National University, Daejeon, Korea. jeikim@cnu.ac.kr
- KMID: 1808483
- DOI: http://doi.org/10.3988/jcn.2005.1.2.134
Abstract
- BACKGROUND AND PURPOSE
The effect of low molecular weight heparin (LMWH) in the management of cerebral venous thrombosis (CVT) remains unclear. The present study was performed to determine the safety and feasibility of subcutaneous LMWH, with particular attention to hemorrhagic conversions.
METHODS
LMWH (nadroparin, 7,500 ICU, every 12 hours) was administered subcutaneously for 14 days to 12 patients diagnosed with CVT. Initial clinical manifestations, etiologies and the clinical courses after LMWH treatment were also evaluated. Possible hemorrhagic side effects, including aggravation of the initial hemorrhage and/or newly developed-hemorrhagic conversions were monitored by image analysis.
RESULTS
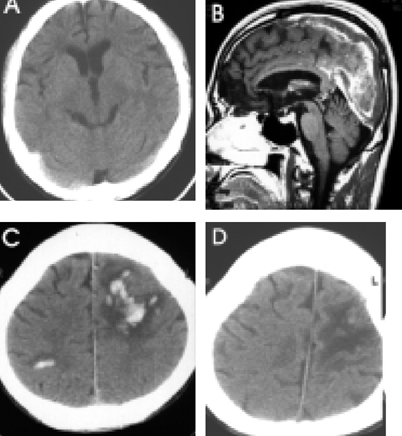
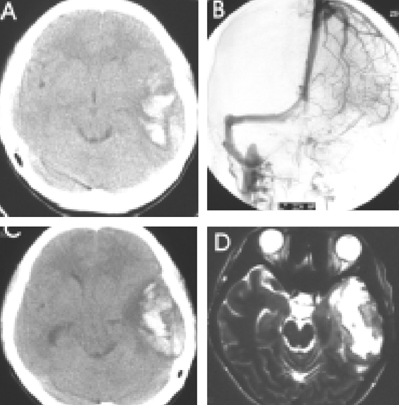
Headaches and convulsive movements were frequent presenting symptoms for CVT. Clinical improvement was usually observed within 2 to 8 days after LMWH. Symptom stabilization was observed within 4 to 60 days. Neither clinical aggravations, nor newly developed parenchymal lesions were observed during LMWH maintenance. Associated parenchymal lesions were observed in 9 of the 12 patients, 5 of which manifested with hemorrhagic conversion, as detected by image analysis. However, no clinical and radiologic aggravation was noted in these 5 patients.
CONCLUSIONS
Our results suggest that LMWH may be safe and feasible in the management of CVT.
MeSH Terms
Figure
Cited by 1 articles
-
Spontaneous Carotid Cavernous Fistula in a Case with Protein S Deficiency that Newly Developed Ophthalmoplegia after Embolization
Chi Kyung Kim, Je-Young Shin, Jun-Young Chang, Seung-Hoon Lee
J Clin Neurol. 2011;7(3):164-167. doi: 10.3988/jcn.2011.7.3.164.
Reference
-
1. Perkin GD. Cerebral venous thrombosis: developments in imaging and treatment. J Neurol Neurosurg Psychiatry. 1995. 59:1–3.
Article2. Bousser M-G, Barnett HJM. Mohr JP, Choi DW, Grotta JC, Weir B, Wolf AP, editors. Cerebral venous thrombosis. Stroke: Pathophysiology, Diagnosis, and Management. 2004. 3rd ed. Philadelphia: Churchill Livingstone;301–325.
Article3. Einhaupl KM, Villringer A, Meister W, Mehraein S, Garner C, Pellkofer M, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991. 338:597–600.
Article4. Stam J, de Bruijn S, deVeber G. Anticoagulation for cerebral sinus thrombosis. Stroke. 2003. 34:1054–1055.
Article5. DiNubile MJ. Septic thrombosis of the cavernous sinuses. Arch Neurol. 1988. 45:567–572.
Article6. Feldenzer JA, Bueche MJ, Venes JL, Gebarski SS. Superior sagittal sinus thrombosis with infarction in sickle cell trait. Stroke. 1987. 18:656–660.
Article7. Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997. 337:688–698.
Article8. Bergqvist D, Burmark US, Flordal PA, Frisell J, Hallbook T, Hedberg M, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995. 82:496–501.
Article9. Torholm C, Broeng L, Jorgensen PS, Bjerregaard P, Josephsen L, Jorgensen PK, et al. Thromboprophylaxis by low-molecular-weight heparin in elective hip surgery. A placebo controlled study. J Bone Joint Surg Br. 1991. 73:434–438.
Article10. Lensing AW, Prins MH, Davidson BL, Hirsh J. Treatment of deep venous thrombosis with low molecular-weight heparins. A meta-analysis. Arch Intern Med. 1995. 155:601–607.
Article11. Kher A, Samama MM. Primary and secondary prophylaxis of venous thromboembolism with low molecular-weight heparins: prolonged thromboprophylaxis, an alternative to vitamin K antagonists. J Thromb Haemost. 2005. 3:473–481.
Article12. SYNERGY Executive Committee. Superior Yield of the New strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa inhibitors. The SYNERGY trial: study design and rationale. Am Heart J. 2002. 143:952–960.13. Blazing MA, de Lemos JA, White HD, Fox KA, Verheugt FW, Ardissino D, et al. Safety and efficacy of enoxaparin vs unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA. 2004. 292:55–64.
Article14. Biller J, Love BB. Bradely WG, Daroff RB, Fenichel GH, Jankovic J, editors. Vascular disease of the venous system. Neurology in Clinical Practice. 2005. 4th ed. Philadelphia: Elsevier Inc.;1243–1245.15. Brucker AB, Vollert-Rogenhofer H, Wagner M, Stieglbauer K, Felber S, Trenkler J, et al. Heparin treatment in acute cerebral sinus venous thrombosis: a retrospective clinical and MR analysis of 42 cases. Cerebrovasc Dis. 1998. 8:331–337.
Article16. Ameri A, Bousser MG. Cerebral venous thrombosis. Neurol Clin. 1992. 10:87–111.
Article17. Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004. 140:175–183.
Article18. Siragusa S, Cosmi B, Piovella F, Hirsh J, Ginsberg JS. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med. 1996. 100:269–277.
Article19. Leizorovicz A, Mismetti P. Preventing venous thromboembolism in medical patients. Circulation. 2004. 110:IV13–IV19.
Article20. de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999. 30:484–488.
Article21. Stam J, Lensing AW, Vermeulen M, Tijssen JG. Heparin treatment for cerebral venous and sinus thrombosis. Lancet. 1991. 338:1154.
Article22. Enevoldson TP, Russell RWR. Heparin treatment in sinus venous thrombosis. Lancet. 1991. 338:1153–1154.
Article23. Das P, Moliterno DJ. Fractionating heparins and their clinical trial data--something for everyone. JAMA. 2004. 292:101–103.
Article24. Hirsh J. Heparin. N Engl J Med. 1991. 324:1565–1574.
Article25. van den Belt AG, Bossuyt PM, Prins MH, Gallus AS, Buller HR. Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis--an economic evaluation. TASMAN Study Group. Thromb Haemost. 1998. 79:259–263.
Article26. Rodger M, Bredeson C, Wells PS, Beck J, Kearns B, Huebsch LB. Cost-effectiveness of low-molecular-weight heparin and unfractionated heparin in treatment of deep vein thrombosis. CMAJ. 1998. 159:931–938.27. Iorio A, Guercini F, Pini M. Low-molecular-weight heparin for the long-term treatment of symptomatic venous thromboembolism: meta-analysis of the randomized comparisons with oral anticoagulants. J Thromb Haemost. 2003. 1:1906–1913.
Article28. Ockelford PA, Patterson J, Johns AS. A double-blind randomized placebo controlled trial of thromboprophylaxis in major elective general surgery using once daily injections of a low molecular weight heparin fragment (Fragmin). Thromb Haemost. 1989. 62:1046–1049.
Article29. Lindmarker P, Holmstrom M, Granqvist S, Johnsson H, Lockner D. Comparison of once-daily subcutaneous Fragmin with continuous intravenous unfractionated heparin in the treatment of deep vein thrombosis. Thromb Haemost. 1994. 72:186–190.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unilateral Thrombosis of a Deep Cerebral Vein Associated with Transient Unilateral Thalamic Edema
- Successful Treatment of Aortic Thrombosis after Umbilical Catheterization with Low-Molecular-Weight Heparin
- Successful Low Molecular Weight Heparin Treatment for the Global Alteration of Cortical Venous Drainage Developed after Intracranial Operation
- Cerebral Venous Thrombosis Presenting in Pregnancy with Thrombocytosis and Janus Kinase 2 Valine-to-Phenylalanine Mutation
- Portal vein thrombosis that successfully treated with low molecular weight heparin in acute pancreatitis