Endocrinol Metab.
2013 Mar;28(1):70-75. 10.3803/EnM.2013.28.1.70.
Olanzapine-Induced Diabetic Ketoacidosis and Neuroleptic Malignant Syndrome with Rhabdomyolysis: A Case Report
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea. sjyoo@catholic.ac.kr
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Psychiatry, Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea.
- KMID: 1805950
- DOI: http://doi.org/10.3803/EnM.2013.28.1.70
Abstract
- Atypical antipsychotics have replaced conventional antipsychotics in the treatment of schizophrenia because they have less of a propensity to cause undesirable neurologic adverse events including extrapyramidal symptoms, tardive dyskinesia, and neuroleptic malignant syndrome (NMS). However, atypical antipsychotics have been known to result in various metabolic complications such as impaired glucose tolerance, diabetes and even diabetic ketoacidosis (DKA). In addition, a number of NMS cases have been reported in patients treated with atypical antipsychotics, although the absolute incidence of neurologic side effects is currently significantly low. Here, we report a patient who simultaneously developed DKA, acute renal failure and NMS with rhabdomyolysis after olanzapine treatment. Olanzapine-induced metabolic complications and NMS were dramatically improved with cessation of the olanzapine treatment and initiation of supportive management including fluid therapy, hemodialysis, and intensive glycemic control using insulin. At short-term follow-up, insulin secretion was markedly recovered as evidenced by a restoration of serum C-peptide level, and the patient no longer required any hypoglycemic medications. Despite the dramatic increase in the use of atypical antipsychotics treatment, individualized treatments along with careful monitoring may be prudent for high risk or vulnerable patients in order to avoid the development of metabolic side effects.
MeSH Terms
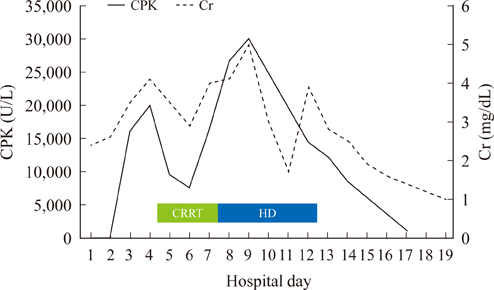
Figure
Cited by 1 articles
-
Brief Review of Articles in '
Endocrinology and Metabolism ' in 2013
Won-Young Lee
Endocrinol Metab. 2014;29(3):251-256. doi: 10.3803/EnM.2014.29.3.251.
Reference
-
1. Kwak YT, Koo MS. Olanzapine-induced neuroletpic malignant syndrome. J Korean Neurol Assoc. 2000. 18:249–251.2. Jeon YJ, Lee SH, Jang SN, Kim ES, Min JY, Kim JH, Hong SH, Cho JH, Kwon HS, Yoon KH, Cha BY, Son HY. Diabetic ketoacidosis in a patient with long-term clozapine therapy. J Korean Endocr Soc. 2007. 22:376–380.3. Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology (Berl). 1996. 124:2–34.4. Fertig MK, Brooks VG, Shelton PS, English CW. Hyperglycemia associated with olanzapine. J Clin Psychiatry. 1998. 59:687–689.5. Wilson DR, D'Souza L, Sarkar N, Newton M, Hammond C. New-onset diabetes and ketoacidosis with atypical antipsychotics. Schizophr Res. 2003. 59:1–6.6. Kim YR, Kwon YJ, Park IJ, Jung HY, Woo KM, Cho MH. Body weight, body mass index, plasma leptin, insulin and fasting glucose level in schizophrenic patients receiving olanzapine. J Korean Neuropsychiatr Assoc. 2001. 40:1240–1246.7. Lee JS, Kim JY, Ahn JH, Kim CY. Diabetic ketoacidosis in a schizophrenic patient treated with olanzapine: a case report. J Korean Neuropsychiatr Assoc. 2005. 44:116–119.8. Misawa F, Miyaji S, Fuji Y, Miyata R, Obi K, Koshiishi F, Shimada H, Fukuda M, Koizumi T. The incidence of hyperglycemia in patients treated with olanzapine. J Clin Psychiatry. 2004. 65:443–444.9. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993. 77:185–202.10. Khaldi S, Kornreich C, Choubani Z, Gourevitch R. Neuroleptic malignant syndrome and atypical antipsychotics: a brief review. Encephale. 2008. 34:618–624.11. Caroff S, Rosenberg H, Gerber JC. Neuroleptic malignant syndrome and malignant hyperthermia. J Clin Psychopharmacol. 1983. 3:120–121.12. May DC, Morris SW, Stewart RM, Fenton BJ, Gaffney FA. Neuroleptic malignant syndrome: response to dantrolene sodium. Ann Intern Med. 1983. 98:183–184.13. Filice GA, McDougall BC, Ercan-Fang N, Billington CJ. Neuroleptic malignant syndrome associated with olanzapine. Ann Pharmacother. 1998. 32:1158–1159.14. Srivastava A, Borkar HA, Chandak S. Olanzapine-induced neuroleptic malignant syndrome in a patient with paranoid schizophrenia. Psychiatry Clin Neurosci. 2009. 63:119–121.15. Baumgart U, Schmid R, Spiessl H. Olanzapine-induced acute rhabdomyolysis: a case report. Pharmacopsychiatry. 2005. 38:36–37.16. Wehring HJ, Kelly DL, Love RC, Conley RR. Deaths from diabetic ketoacidosis after long-term clozapine treatment. Am J Psychiatry. 2003. 160:2241–2242.17. Ader M, Kim SP, Catalano KJ, Ionut V, Hucking K, Richey JM, Kabir M, Bergman RN. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes. 2005. 54:862–871.18. Hardy TA, Meyers AL, Yu J, Shankar SS, Steinberg HO, Porksen NK. Acute insulin response and beta-cell compensation in normal subjects treated with olanzapine or risperidone for 2 weeks. Diabetes Care. 2007. 30:157–158.19. Sowell MO, Mukhopadhyay N, Cavazzoni P, Shankar S, Steinberg HO, Breier A, Beasley CM Jr, Dananberg J. Hyperglycemic clamp assessment of insulin secretory responses in normal subjects treated with olanzapine, risperidone, or placebo. J Clin Endocrinol Metab. 2002. 87:2918–2923.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Olanzapine-induced Neuroletpic Malignant Syndrome
- Rhabdomyolysis and Neuroleptic Malignant Syndrome Associated with Very Low-dose Antipsychotics in Children and Adolescent
- A Case of Acute Renal Failure due to Rhabdomyolysis Associated with Neuroleptic Malignant Syndrome
- A Case of Diabetic Ketoacidosis by Olanzapine
- Acute Renal Failure, a Sequela of the Neuroleptic Malignant Syndrome