J Korean Med Sci.
2010 Dec;25(12):1748-1753. 10.3346/jkms.2010.25.12.1748.
Safety and Effectiveness of Central Venous Catheterization in Patients with Cancer: Prospective Observational Study
- Affiliations
-
- 1Department of Internal Medicine, Division of Hematology & Oncology, Soonchunhyang University College of Medicine, Bucheon, Korea. dshong@schbc.ac.kr
- KMID: 1792898
- DOI: http://doi.org/10.3346/jkms.2010.25.12.1748
Abstract
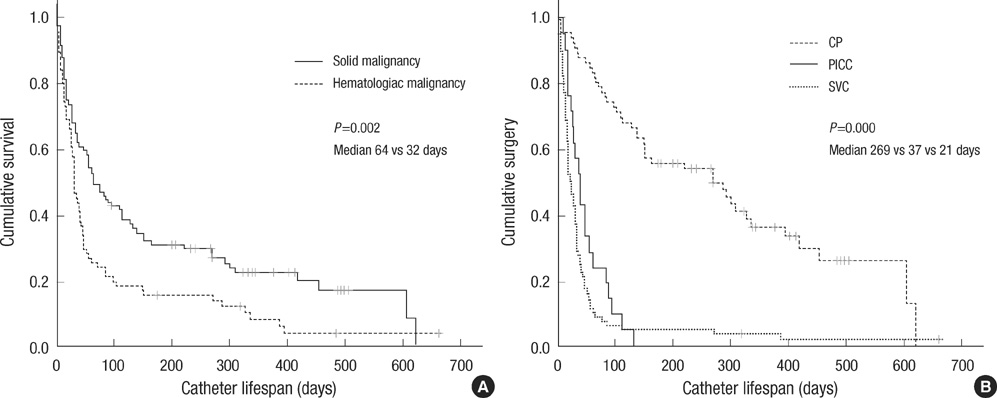
- This study investigated the safety and effectiveness of each type of central venous catheters (CVC) in patients with cancer. We prospectively enrolled patients with cancer who underwent catherization involving a subclavian venous catheter (SVC), peripherally inserted central venous catheter (PICC), or chemo-port (CP) in our department. From March 2007 to March 2009, 116 patients underwent 179 episodes of catherization. A SVC was inserted most frequently (46.4%). Fifty-four complications occurred (30.1%): infection in 23 cases, malpositioning or migration of the tip in 18 cases, thrombosis in eight cases, and bleeding in five cases. Malpositioning or migration of the tip occurred more frequently with a PICC (P<0.001); infection occurred more often with a tunneled catheter (P=0.028) and was observed more often in young patients (P=0.023). The catheter life span was longer for patients with solid cancer (P=0.002) than for those with hematologic cancer, with a CP (P<0.001) than a PICC or SVC, and for an indwelling catheter with image guidance (P=0.014) than a blind procedure. In conclusion, CP is an effective tool for long term use and the fixation of tip is important for the management of PICC.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Catheter-Related Infections/epidemiology/etiology
Catheterization, Central Venous/*adverse effects
Catheterization, Peripheral/adverse effects
Equipment Failure
Female
Hemorrhage/epidemiology/etiology
Humans
Male
Middle Aged
Neoplasms/*drug therapy
Prospective Studies
Risk Factors
Thrombosis/epidemiology/etiology
Figure
Cited by 1 articles
-
A Case-Control Study to Identify Risk Factors for Totally Implantable Central Venous Port-Related Bloodstream Infection
Guk Jin Lee, Sook Hee Hong, Sang Young Roh, Sa Rah Park, Myung Ah Lee, Hoo Geun Chun, Young Seon Hong, Jin Hyoung Kang, Sang Il Kim, Youn Jeong Kim, Ho Jong Chun, Jung Suk Oh
Cancer Res Treat. 2014;46(3):250-260. doi: 10.4143/crt.2014.46.3.250.
Reference
-
1. Scott WL. Central venous catheters. An overview of Food and Drug Administration activities. Surg Oncol Clin N Am. 1995. 4:377–393.2. Mansfield PF, Hohn DC, Fornage BD, Gregurich MA, Ota DM. Complications and failures of subclavian-vein catheterization. N Engl J Med. 1994. 331:1735–1738.
Article3. Yildizeli B, Laçin T, Batirel HF, Yüksel M. Complications and management of long-term central venous access catheters and ports. J Vasc Access. 2004. 5:174–178.
Article4. Cortelezzia A, Fracchiolla NS, Maisonneuve P, Moia M, Luchesini C, Ranzi ML, Monni P, Pasquini MC, Lambertenghi-Deliliers G. Central venous catheter-related complications in patients with hematological malignancies: a retrospective analysis of risk factors and prophylactic measures. Leuk Lymphoma. 2003. 44:1495–1501.5. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003. 348:1123–1133.
Article6. Vescia S, Baumgärtner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, Harbeck N. Management of venous port systems in oncology: a review of current evidence. Ann Oncol. 2008. 19:9–15.
Article7. Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008. 58:323–346.
Article8. Kim JT, Oh TY. Clinical review of totally implantable venous catheter. Korean J Thorac Cardiovasc Surg. 2007. 40:691–695.9. Cho SG, Kim SH, Song HH, Song SH, Lee KH, Chung DY, Lee HJ, Kim SH, Kim KT, Kim CC. Radiologic placement of subcutaneous infusion ports in cancer patients: analysis of 45 cases. J Korean Cancer Assoc. 2000. 32:1115–1121.10. Ryder MA. Peripheral access options. Surg Oncol Clin N Am. 1995. 4:395–427.
Article11. Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, Mc-Dougall C, Wilcox MH. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2007. 65:Suppl 1. S1–S64.
Article12. King MM, Rasnake MS, Rodriguez RG, Riley NJ, Stamm JA. Peripherally inserted central venous catheter-associated thrombosis: retrospective analysis of clinical risk factors in adult patients. South Med J. 2006. 99:1073–1077.
Article13. Howell PB, Walters PE, Donowitz GR, Farr BM. Risk factors for infection of adult patients with cancer who have tunnelled central venous catheters. Cancer. 1995. 75:1367–1375.
Article14. Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966. 64:328–340.
Article15. Samaras P, Dold S, Braun J, Kestenholz P, Breitenstein S, Imhof A, Renner C, Stenner-Liewen F, Pestalozzi BC. Infectious port complications are more frequent in younger patients with hematologic malignancies than in solid tumor patients. Oncology. 2008. 74:237–244.
Article16. Camargo LF, Marra AR, Büchele GL, Sogayar AM, Cal RG, de Sousa JM, Silva E, Knobel E, Edmond MB. Double-lumen central venous catheters impregnated with chlorhexidine and silver sulfadiazine to prevent catheter colonisation in the intensive care unit setting: a prospective randomised study. J Hosp Infect. 2009. 72:227–233.
Article17. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008. 29:947–950.
Article18. Bern MM, Lokich JJ, Wallach SR, Bothe A Jr, Benotti PN, Arkin CF, Greco FA, Huberman M, Moore C. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990. 112:423–428.19. Monreal M, Alastrue A, Rull M, Mira X, Muxart J, Rosell R, Abad A. Upper extremity deep venous thrombosis in cancer patients with venous access devices prophylaxis with a low molecular weight heparin (Fragmin). Thromb Haemost. 1996. 75:251–253.20. Couban S, Goodyear M, Burnell M, Dolan S, Wasi P, Barnes D, Macleod D, Burton E, Andreou P, Anderson DR. Randomized Placebo-Controlled Study of Low-Dose Warfarin for the Prevention of Central Venous Catheter-Associated Thrombosis in Patients With Cancer. J Clin Oncol. 2005. 23:4063–4069.
Article21. Heaton DC, Han DY, Inder A. Minidose (1 mg) warfarin as prophylaxis for central vein catheter thrombosis. Intern Med J. 2002. 32:84–88.
Article22. Young AM, Billingham LJ, Begum G, Kerr DJ, Hughes AI, Rea DW, Shepherd S, Stanley A, Sweeney A, Wilde J, Wheatley K. WARP Collaborative Group, UK. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009. 373:567–574.
Article23. Svoboda P, Barton RP, Barbarash OL, Butylin AA, Jacobs BR, Lata J, Haire WD, Jaff MR, Firszt CM, Mouginis TL, Schuerr DM, Schulz GA, Schwartz LB, El-Shahawy MA. Recombinant urokinase is safe and effective in restoring patency to occluded central venous access devices: a multiple-center, international trial. Crit Care Med. 2004. 32:1990–1996.
Article24. Schindler J, Bona RD, Chen HH, Feingold JM, Edwards RL, Tutschka PJ, Bilgrami S. Regional thrombolysis with urokinase for central venous catheter-related thrombosis in patients undergoing high-dose chemotherapy with autologous blood stem cell rescue. Clin Appl Thromb Hemost. 1999. 5:25–29.
Article25. Grove JR, Pevec WC. Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. 2000. 11:837–840.
Article26. Loewenthal MR, Dobson PM, Starkey RE, Dagg SA, Petersen A, Boyle MJ. The peripherally inserted central catheter (PICC): a prospective study of its natural history after cubital fossa insertion. Anaesth Intensive Care. 2002. 30:21–24.
Article27. Gabriel J. PICC securement: minimising potential complications. Nurs Stand. 2001. 15:42–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inadvertent Arterial Catheterization of Central Venous Catheter: A Case Report
- Mediastinal and Intrapleural Hydrothorax Following Subclavian Central Venous Catheterization - A case report
- Hydrohemothorax following Subclavian Vein Catheterization
- Hemomediastinum Caused by Subclavian Catheterization : A case report
- A Safe Method of Central Venous Catheterization by Peripheral Venous Cutdown in Infants