Korean J Gastroenterol.
2013 Oct;62(4):248-252. 10.4166/kjg.2013.62.4.248.
A Case of Pleomorphic Liposarcoma in a Patient with Crohn's Disease Taking Azathioprine
- Affiliations
-
- 1Department of Internal Medicine, National Police Hospital, Seoul, Korea. tgrw100@hanmail.net
- 2Department of Thoracic and Cardiovascular Surgery, National Police Hospital, Seoul, Korea.
- 3Department of Pathology, National Police Hospital, Seoul, Korea.
- KMID: 1792745
- DOI: http://doi.org/10.4166/kjg.2013.62.4.248
Abstract
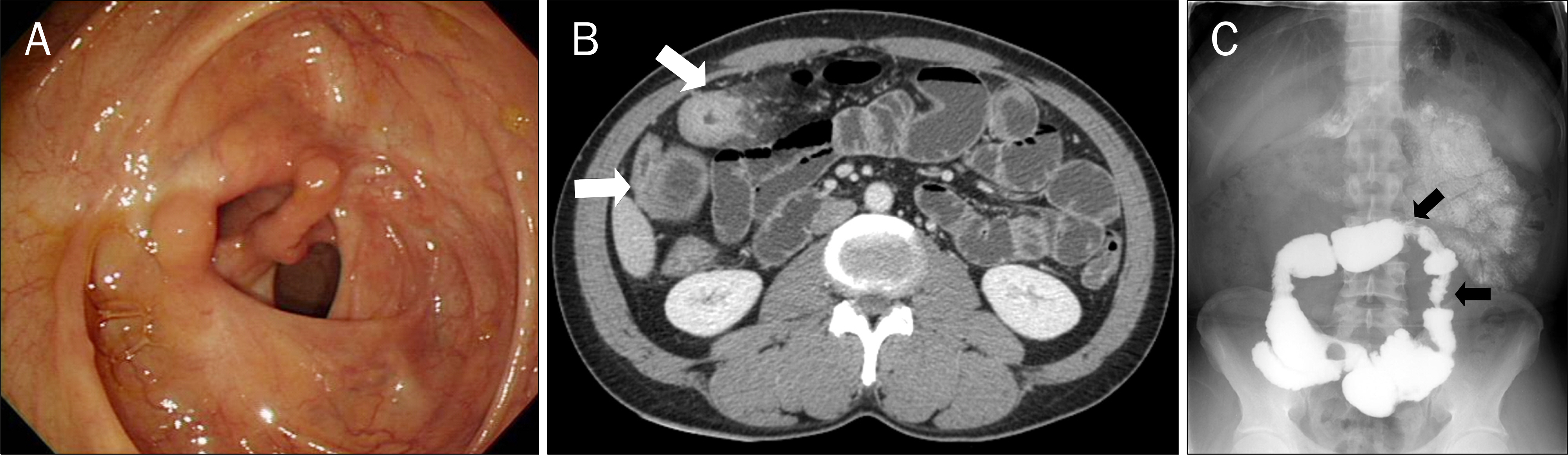
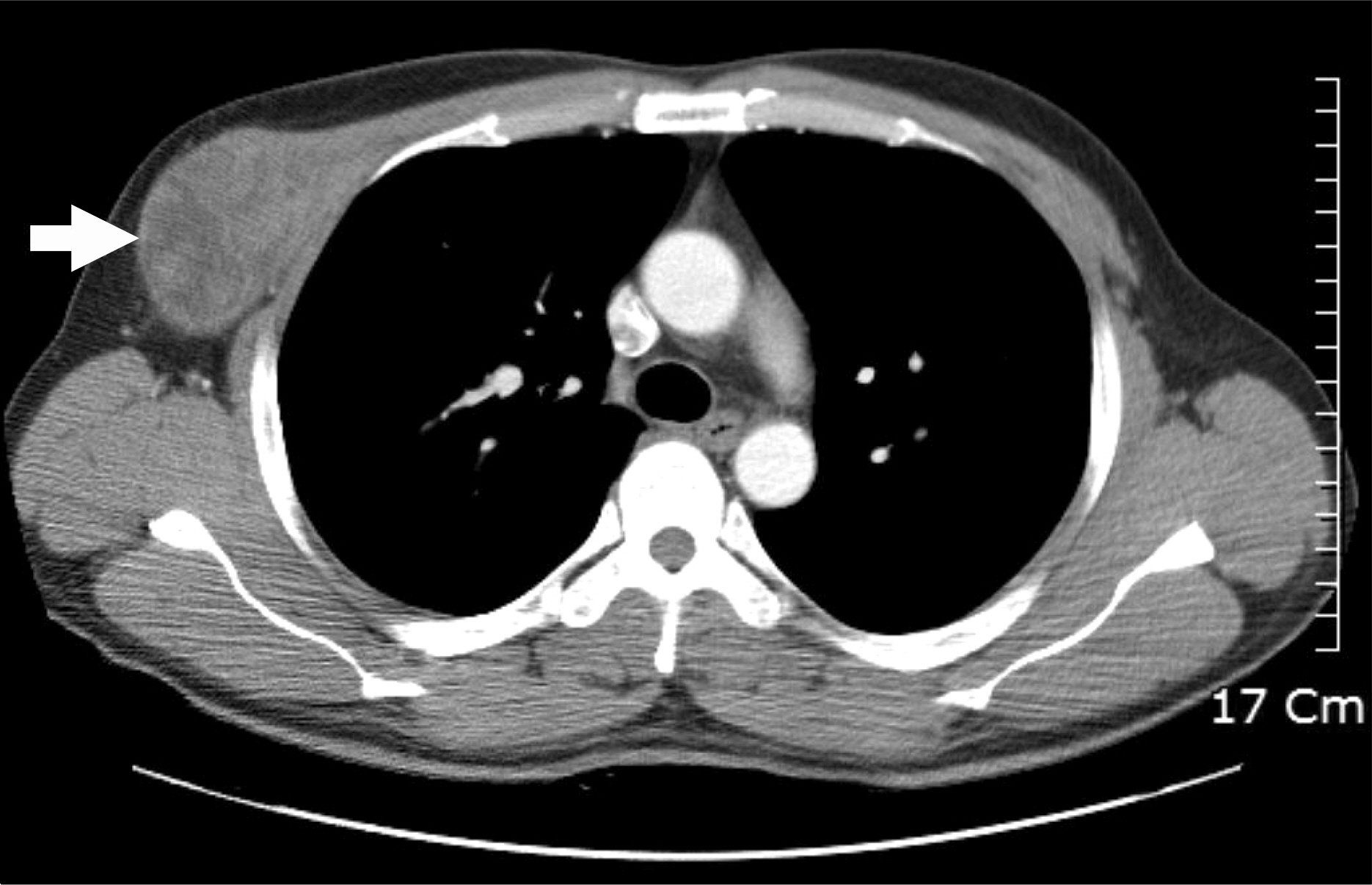
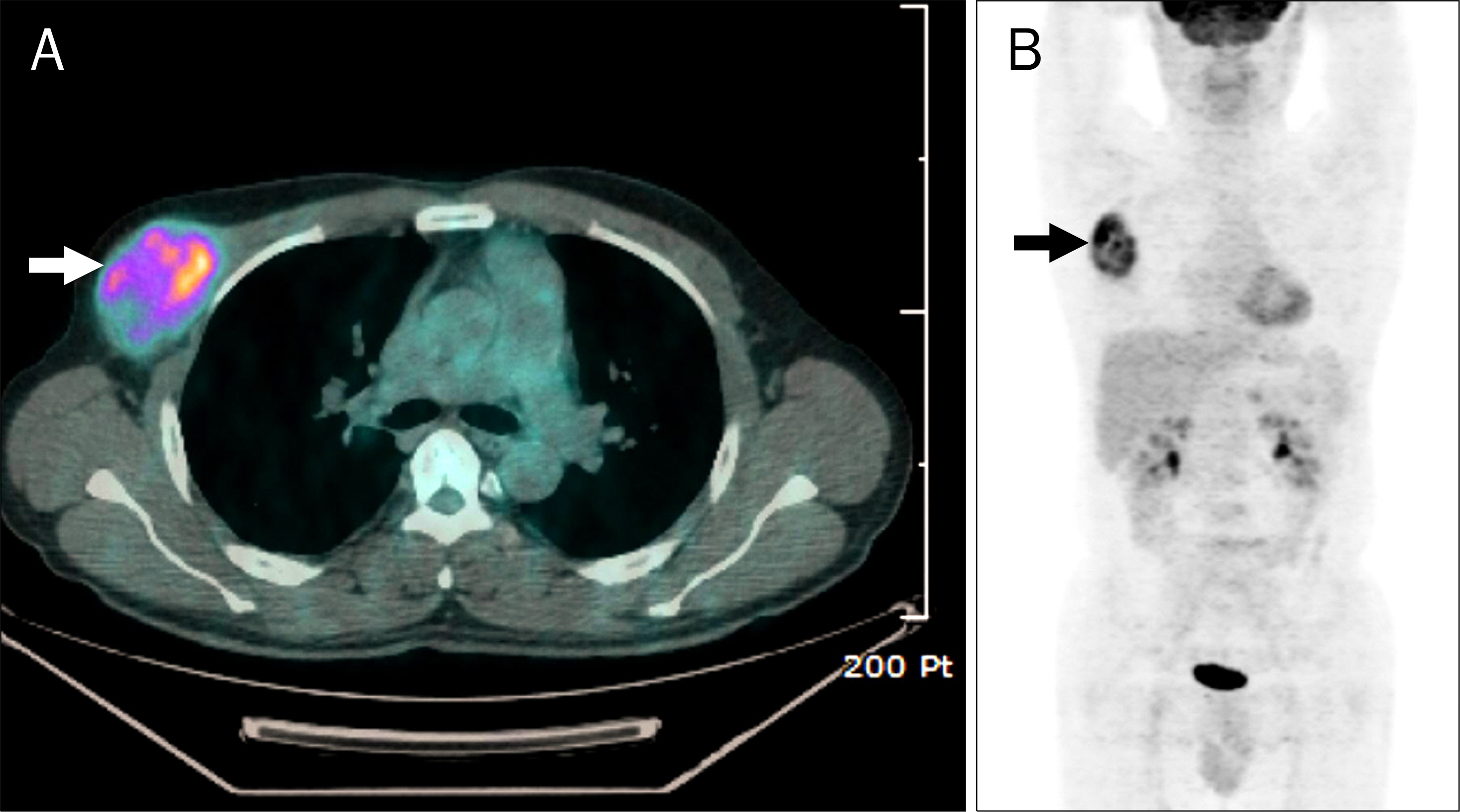
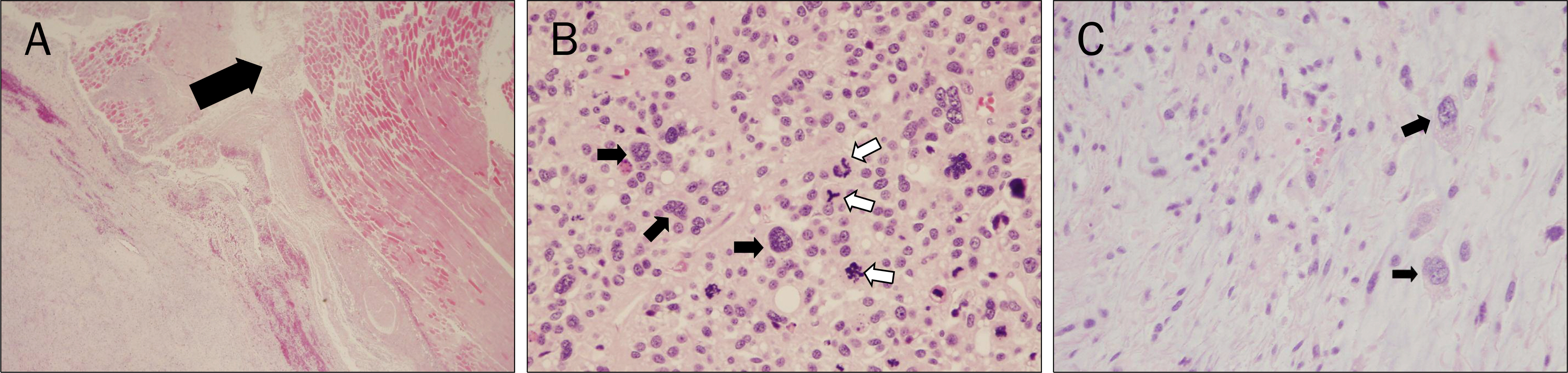
- Azathioprine is frequently used for the treatment of inflammatory bowel diseases (IBD) such as Crohn's disease and ulcerative colitis. Lymphomas, squamous cell carcinomas, and undifferentiated pleomorphic sarcomas have been reported among patients receiving azathioprine therapy. Herein, we report a case of pleomorphic liposarcoma of chest wall which occurred in a 44-year-old man with Crohn's disease taking azathioprine. He was diagnosed with Crohn's disease 3 years ago after suffering from abdominal pain and hematochezia for 12 years. He had been taking 50 mg of azathioprine per day for 23 months when he visited the thoracic and cardiovascular surgery clinic due to right chest palpable mass that had rapidly grown during the past 2 months. Excisional biopsy was performed and the mass was diagnosed as pleomorphic liposarcoma. Therefore, he underwent radical excision of the right chest wall mass, which measured 11.0x6.5 cm in size. He is scheduled to receive radiation therapy and chemotherapy.
Keyword
MeSH Terms
-
Adult
Azathioprine/*therapeutic use
Colonoscopy
Combined Modality Therapy
Crohn Disease/complications/*drug therapy
Fluorodeoxyglucose F18/diagnostic use
Humans
Immunosuppressive Agents/*therapeutic use
Liposarcoma/complications/*pathology/surgery
Male
Positron-Emission Tomography
Radiopharmaceuticals/diagnostic use
Tomography, X-Ray Computed
Azathioprine
Fluorodeoxyglucose F18
Immunosuppressive Agents
Radiopharmaceuticals
Figure
Cited by 1 articles
-
Effect of Immunomodulators and Biologic Agents on Malignancy in Patients with Inflammatory Bowel Disease
Jee Hyun Kim, Ji Won Kim
Korean J Gastroenterol. 2017;70(4):162-168. doi: 10.4166/kjg.2017.70.4.162.
Reference
-
References
1. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002; 50:485–489.
Article2. Ye BD, Yang SK, Shin SJ, et al. IBD Study Group of the Korean Association for the Study of the Intestinal Diseases. Guidelines for the management of Crohn's disease. Korean J Gastroenterol. 2012; 59:141–179.
Article3. Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther. 2010; 32:119–130.
Article4. Cetin B, Büyükberber S, Yilmaz IB, Yildiz R, Coş kun U, Benekl M. Kaposi's sarcoma in patients with ulcerative colitis receiving immunosuppressive drugs: report of a case. Turk J Gastroenterol. 2011; 22:621–625.
Article5. Sood R, Daw HA. Pleomorphic malignant histiocytoma: a rare skin cancer in a patient on azathioprine for ulcerative colitis. BMJ Case Rep. 2012; 2012:DOI: doi: 10.1136/bcr.03.2012.5957.
Article6. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.
Article7. Lee HJ, Yang SK, Kim KJ, et al. The safety and efficacy of azathioprine/6-mercaptopurine in the treatment of Korean patients with Crohn's disease. Intest Res. 2009; 7:22–31.8. Swann PF, Waters TR, Moulton DC, et al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996; 273:1109–1111.
Article9. Fraser AG, Orchard TR, Robinson EM, Jewell DP. Longterm risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. 2002; 16:1225–1232.
Article10. Connel WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Longterm neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994; 343:1249–1252.11. Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989; 111:641–649.
Article12. Beaugerie L, Brousse N, Bouvier AM, et al. CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009; 374:1617–1625.
Article13. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005; 54:1121–1125.
Article14. Kim JY, Lee J, Lee SE, et al. The type and incidence of malignancy in 1500 renal transplant recipients at Kangnam St. Mary's Hospital. Korean J Med. 2007; 73:67–75.15. Kim HS, Lee J, Yi SY, et al. Liposarcoma: exploration of clinical prognostic factors for risk based stratification of therapy. BMC Cancer. 2009; 9:205.
Article16. Fletcher CDM, Unni KK, Mertens F. World Health Organization classification of tumors: pathology and genetics of tumours of soft tissue and bone. 4th ed.Lyon: IARC Press;2002.17. Kim HC, Nam SW, Cho YK, et al. A case of non-Hodgkin's lymphoma in a patient with Crohn's disease. Korean J Gastroenterol. 2006; 47:233–237.18. Park CK, Ju DU, Heo SW, et al. A case of squamous cell carcinoma of the breast in a patient with Crohn's disease taking azathioprine. Korean J Gastroenterol. 2012; 60:373–376.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Azathioprine-Induced Non-Cirrhotic Portal Hypertension in a Patient with Crohn’s Disease
- Determining the Dose of Azathioprine Based on the Lower Limit of Leukocyte Count in Patients with Crohn's Disease
- A Case of Squamous Cell Carcinoma of the Breast in a Patient with Crohn's Disease Taking Azathioprine
- A Case of Pleomorphic Lipoma in the Scalp
- Retroperitoneal Pleomorphic Liposarcoma Mimicking Adrenal Cancer in F-18 FDG PET/CT