J Korean Med Assoc.
2012 Sep;55(9):861-868. 10.5124/jkma.2012.55.9.861.
The role of the KIDS for enhancing drug safety and risk management in Korea
- Affiliations
-
- 1Korea Institute of Drug Safety and Risk Management, Seoul, Korea. bjpark@drugsafe.or.kr
- 2Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1787249
- DOI: http://doi.org/10.5124/jkma.2012.55.9.861
Abstract
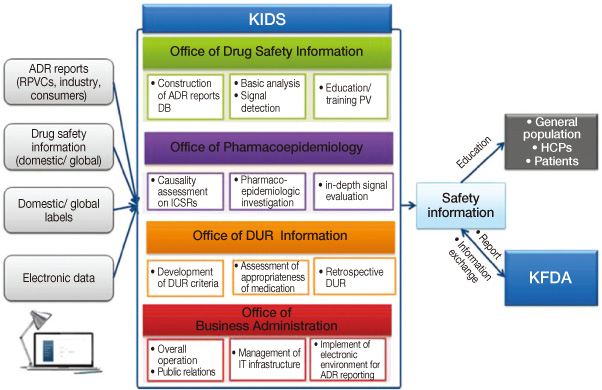
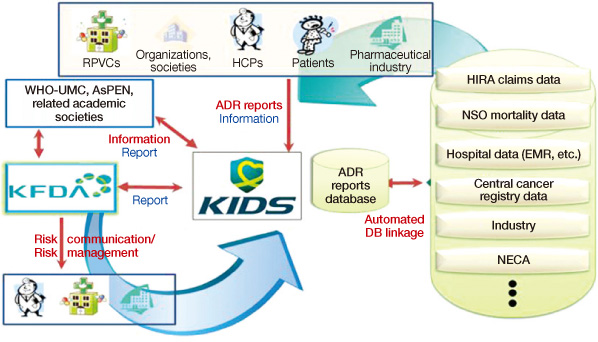
- Pharmacovigilance is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. The Korea Institute of Drug Safety and Risk Management (KIDS) was established in April 2012. The main missions of the KIDS are to collect, analyze, evaluate, and manage adverse drug reaction (ADR) data, develop drug utilization review (DUR) criteria, distribute medication guidelines for health professionals, perform retrospective DUR, and perform causality assessment on ADRs, or signals derived from spontaneous reporting or other sources. The KIDS aims to provide evidence to enhance national health quality through a better drug safety and risk management system in Korea. The purpose of this paper is to suggest possible approaches and the KIDS's role in strengthening the pharmacovigilance system in Korea.
MeSH Terms
Figure
Cited by 1 articles
-
Use of big data for drug safety monitoring and decision making
Sun-Young Jung, Nam-Kyong Choi, Joongyub Lee, Byung-Joo Park
J Korean Med Assoc. 2014;57(5):391-397. doi: 10.5124/jkma.2014.57.5.391.
Reference
-
1. Park BJ. Prospectus of the Korean Society for Pharmacoepidemiology and Risk Management. J Pharmacoepidemiol Risk Manag. 2008. 1:5–7.2. Korea Food and Drug Administration. Pharmacovigilance Research Network. Standard operation procedures for pharmacovigilance research network regional pharmacovigilance centers. 2011. Seoul: Pharmacovigilance Research Network.3. Choi NK, Park BJ. Adverse drug reaction surveillance system in Korea. J Prev Med Public Health. 2007. 40:278–284.
Article4. Jang BW. Pharmaceutical affairs law and administration. 2012. Seoul: SinilBooks;520.5. Uppsala Monitoring Center. Active ICSRS in the WHO global ICSR database per million inhabitants and year [Internet]. 2012. cited 2012 Aug 27. Uppsala: Uppsala Monitoring Center;Available from: http://who-umc.org/graphics/26930.gif.6. Uppsala Monitoring Center. Guidelines for setting up and running a pharmacovigilance centre [Internet]. 2000. cited 2012 Aug 27. Uppsala: Uppsala Monitoring Center;Available from: http://www.who-umc.org/DynPage.aspx?id=105828&mn1=7347&mn2=7259&mn3=7297&mn4=7496.7. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001. 10:483–486.
Article8. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004. 13:519–523.
Article9. Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RM. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998. 54:315–321.
Article10. Seong JM, Choi NK, Ahn SH, Park BJ. Development of signal detection program for adverse drug reaction. J Pharmacoepidemiol Risk Manag. 2011. 4:15–21.11. Council for International Organizations of Medical Sciences Working Group VIII. Practical aspects of signal detection in pharmacovigilance: report of CIOMS Working Group VIII. 2010. Geneva: Council for International Organizations of Medical Sciences.12. Stahl M, Lindquist M, Edwards IR, Brown EG. Introducing triage logic as a new strategy for the detection of signals in the WHO Drug Monitoring Database. Pharmacoepidemiol Drug Saf. 2004. 13:355–363.
Article13. Center for Drug Evaluation and Research (US). Center for Biologics Evaluation and Research (US). Guidance for industry: good pharmacovigilance practices and pharmacoepidemiologic assessment. 2005. Rockville: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research.14. Waller PC, Tilson HH. Stephens MD, Talbot JC, Waller P, editors. Managing drug safety issues with marketed products. Stephens' detection of new adverse drug reactions. 2004. 5th ed. Hoboken: John Wiley & Sons;345–374.
Article15. Loke YK, Price D, Herxheimer A. Cochrane Adverse Effects Methods Group. Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol. 2007. 7:32.
Article16. Hall GC, Sauer B, Bourke A, Brown JS, Reynolds MW, Casale RL. Guidelines for good database selection and use in pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2012. 21:1–10.
Article17. Choi NK, Hahn S, Park BJ. Increase in mortality rate following coprescription of cisapride and contraindicated drugs. Ann Pharmacother. 2007. 41:667–673.
Article18. Park BJ. Drug utilization review. J Pharmacoepidemiol Risk Manag. 2008. 1:13–19.19. Choi NK, Park BJ. Strategy for establishing an effective Korean drug utilization review system. J Korean Med Assoc. 2010. 53:1130–1138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of big data for drug safety monitoring and decision making
- Risk management and principal features of REMS
- Signal Detection for Cardiovascular Adverse Events of DPP-4 Inhibitors Using the Korea Adverse Event Reporting System Database, 2008–2016
- The Enhancing Error Management of Pilot and Line Operation Safety Audit
- Benzodiazepine-Related Cognitive Impairment or Dementia: A Signal Detection Study Using a Case/Non-Case Approach