Clin Orthop Surg.
2013 Dec;5(4):314-320. 10.4055/cios.2013.5.4.314.
Treatment Outcomes of Advanced Stage Malignant Melanoma in Hand and Foot after Amputation in Korean Patients
- Affiliations
-
- 1Department of Orthopaedics, Chonnam National University Hospital, Gwangju, Korea. stjung@jnu.ac.kr
- KMID: 1787035
- DOI: http://doi.org/10.4055/cios.2013.5.4.314
Abstract
- BACKGROUND
A retrospective study was conducted to review the overall survival and treatment outcomes of high grade melanoma in the extremity to explore the clinical features of malignant melanoma of the hand and foot, and the therapeutic efficacies and survival rate after amputation.
METHODS
The clinical data of 30 patients with malignant melanoma of the hand and foot (confirmed by pathological examination), who were admitted and treated in our hospital between 2001 and 2010, were analyzed retrospectively. We analyzed variables affecting overall and disease-free survival.
RESULTS
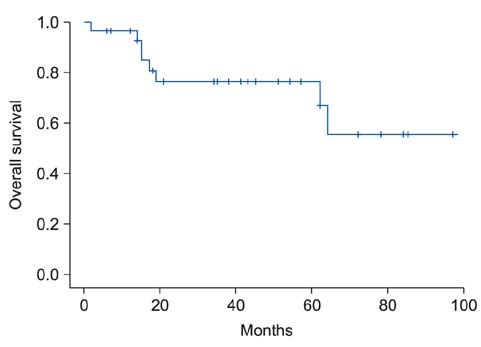
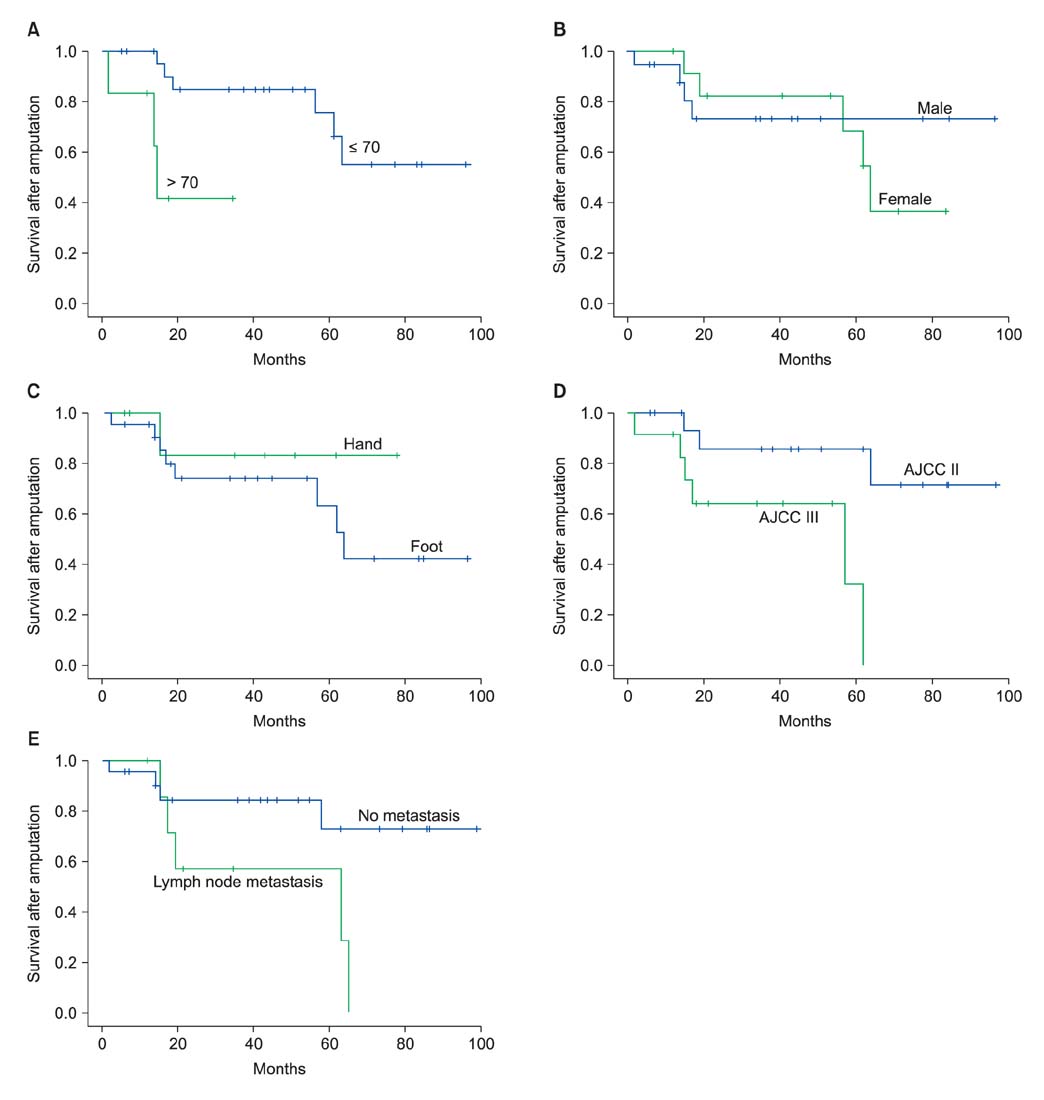
Thirty patients (18 men and 12 women) treated with an amputation procedure for malignant melanoma in the hand or foot constituted the study cohort. The average age of the patients at the time of diagnosis was 58.7 years. Univariate analysis for overall melanoma survival revealed that diagnosis at over 70 years of age, postoperative lymph node metastasis, and location of the tumor were significant prognostic factors. And on the Kaplan-Meier survival curve, old age, American Joint Committee on Cancer stage and postoperative lymph node metastasis showed statistically significant differences in the 5-year survival rate. Also, amputation with aggressive lymph node dissection showed improved long term survival in advanced stage melanoma.
CONCLUSIONS
In Korean melanoma patients, for the treatment of high grade melanomas in the extremities after amputation, early diagnosis and postoperative follow-up for evaluation of lymph node metastasis are critical factors for long-term survival. And by performing lymph node dissection during amputation, we may improve the survival rate in advanced stage melanoma patients.
Keyword
MeSH Terms
-
Aged
*Amputation
Analysis of Variance
Disease-Free Survival
Female
Foot/*surgery
Hand/*surgery
Humans
Kaplan-Meier Estimate
Male
Melanoma/diagnosis/pathology/*surgery
Middle Aged
Neoplasm Staging
Prognosis
Republic of Korea
Retrospective Studies
Skin Neoplasms/diagnosis/pathology/*surgery
Survival Rate
Treatment Outcome
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57(1):43–66.
Article2. Geller AC, Swetter SM, Brooks K, Demierre MF, Yaroch AL. Screening, early detection, and trends for melanoma: current status (2000-2006) and future directions. J Am Acad Dermatol. 2007; 57(4):555–572.
Article3. Garbe C, McLeod GR, Buettner PG. Time trends of cutaneous melanoma in Queensland, Australia and Central Europe. Cancer. 2000; 89(6):1269–1278.4. Tanaka H, Tsukuma H, Tomita S, et al. Time trends of incidence for cutaneous melanoma among the Japanese population: an analysis of Osaka Cancer Registry data, 1964-95. J Epidemiol. 1999; 9:6 Suppl. S129–S135.5. Sng J, Koh D, Siong WC, Choo TB. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009; 61(3):426–432.6. Berwick M, Begg CB, Armstrong BK, et al. Interaction of CDKN2A and sun exposure in the etiology of melanoma in the general population. J Invest Dermatol. 2011; 131(12):2500–2503.7. Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011; 135(7):838–841.8. Waster P, Rosdahl I, Gilmore BF, Seifert O. Ultraviolet exposure of melanoma cells induces fibroblast activation protein-α in fibroblasts: implications for melanoma invasion. Int J Oncol. 2011; 39(1):193–202.9. Bae JM, Kim HO, Park YM. Progression from acral lentiginous melanoma in situ to invasive acral lentiginous melanoma. Ann Dermatol. 2009; 21(2):185–188.10. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27(36):6199–6206.11. Bellew S, Del Rosso JQ, Kim GK. Skin cancer in asians: part 2: melanoma. J Clin Aesthet Dermatol. 2009; 2(10):34–36.12. Lee MW, Koh JK, Kwon KS, et al. Clinical and histopathological study of cutaneous melanoma in Korea. Korean J Dermatol. 2003; 41(1):43–47.13. Moon SE, Cho KH, Hwang JH, Kim JA, Youn JI. A statistical study of cutaneous malignant tumors. Korean J Dermatol. 1998; 36(1):7–15.14. Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000; 88(10):2398–2424.15. Takematsu H, Obata M, Tomita Y, Kato T, Takahashi M, Abe R. Subungual melanoma: a clinicopathologic study of 16 Japanese cases. Cancer. 1985; 55(11):2725–2731.16. Collins RJ. Melanoma in the Chinese of Hong Kong: emphasis on volar and subungual sites. Cancer. 1984; 54(7):1482–1488.17. Park KG, Blessing K, Kernohan NM. Surgical aspects of subungual malignant melanomas: the Scottish Melanoma Group. Ann Surg. 1992; 216(6):692–695.18. Blessing K, Kernohan NM, Park KG. Subungual malignant melanoma: clinicopathological features of 100 cases. Histopathology. 1991; 19(5):425–429.19. Slingluff CL Jr, Vollmer R, Seigler HF. Acral melanoma: a review of 185 patients with identification of prognostic variables. J Surg Oncol. 1990; 45(2):91–98.20. Balch CM, Murad TM, Soong SJ, Ingalls AL, Richards PC, Maddox WA. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer. 1979; 43(3):883–888.21. Balch CM, Soong SJ, Milton GW, et al. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982; 196(6):677–684.22. Pack GT, Oropeza R. Subungual melanoma. Surg Gynecol Obstet. 1967; 124(3):571–582.23. Papachristou DN, Fortner JG. Melanoma arising under the nail. J Surg Oncol. 1982; 21(4):219–222.24. Muchmore JH, Krementz ET, Carter RD, Sutherland CM, Godfrey RS. Regional perfusion for the treatment of subungual melanoma. Am Surg. 1990; 56(2):114–118.25. Baas PC, Hoekstra HJ, Schraffordt Koops H, Oosterhuis JW, Oldhoff J. Isolated regional perfusion in the treatment of subungual melanoma. Arch Surg. 1989; 124(3):373–376.26. Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001; 19(16):3622–3634.27. Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995; 181(3):193–201.28. Leo F, Cagini L, Rocmans P, et al. Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer. 2000; 83(5):569–572.29. Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004; 54(3):131–149.30. Luk NM, Ho LC, Choi CL, Wong KH, Yu KH, Yeung WK. Clinicopathological features and prognostic factors of cutaneous melanoma among Hong Kong Chinese. Clin Exp Dermatol. 2004; 29(6):600–604.