J Korean Med Sci.
2008 Feb;23(1):104-110. 10.3346/jkms.2008.23.1.104.
Analysis of Serum Levels of Anti-Mullerian Hormone, Inhibin B, Insulin-Like Growth Factor-I, Insulin-Like Growth Factor Binding Protein-3, and Follicle-Stimulating Hormone with Respect to Age and Menopausal Status
- Affiliations
-
- 1Department of Nuclear Medicine, College of Medicine, Seoul National University, Seoul, Korea. jkchung@plaza.snu.ac.kr
- 2Tumor Immunity Medical Research Center; College of Medicine, Seoul National University, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, College of Medicine, Seoul National University, Seoul, Korea.
- KMID: 1786854
- DOI: http://doi.org/10.3346/jkms.2008.23.1.104
Abstract
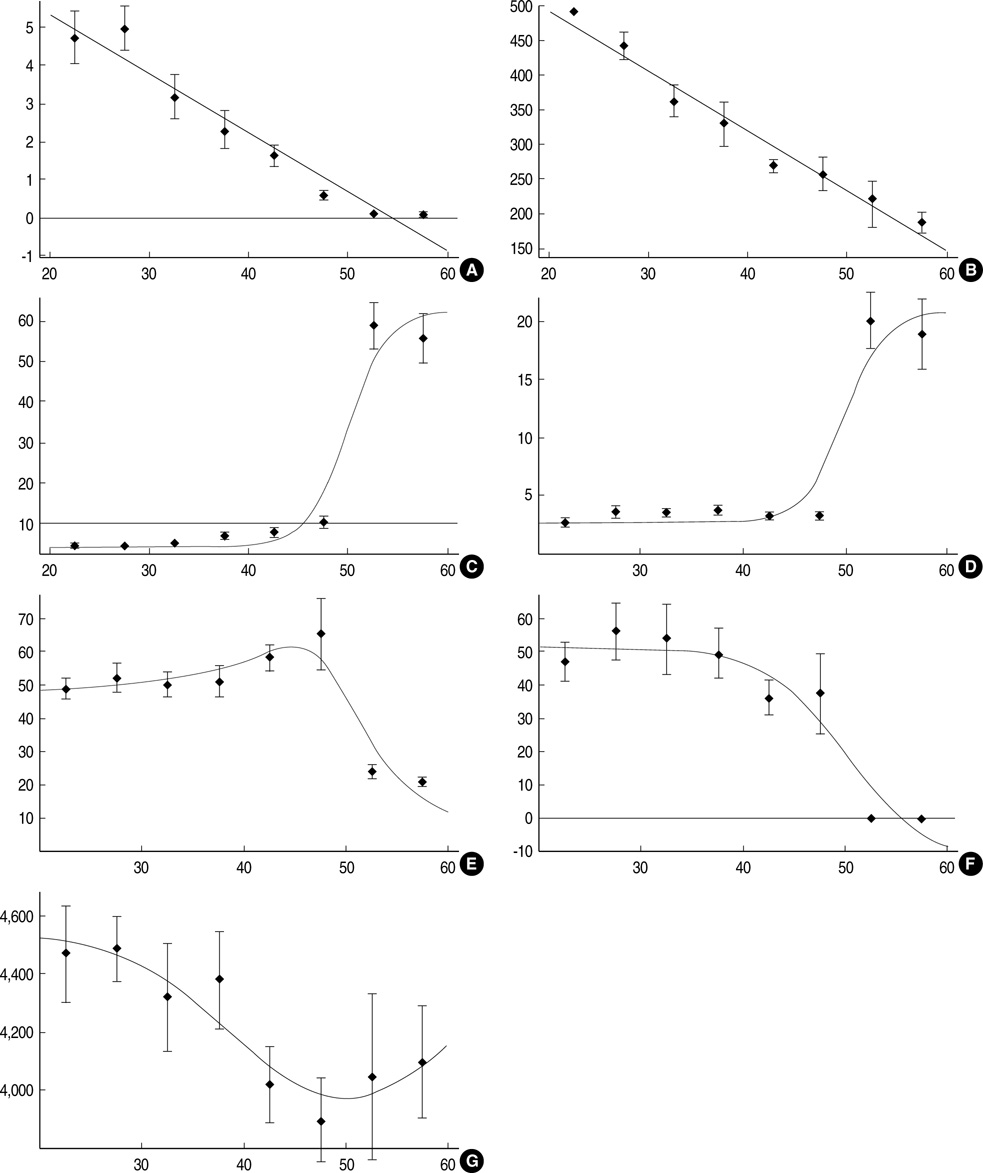
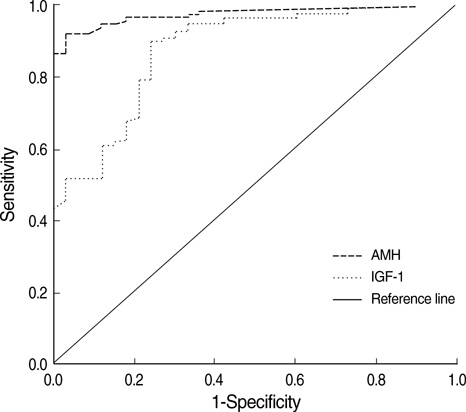
- This study was undertaken to investigate age-dependent and postmenopausal changes in the serum levels of anti-Mullerian hormone (AMH), inhibin B, insulin-like growth factor (IGF)-I, IGF-binding protein-3 (IGFBP-3), and follicle-stimulating hormone (FSH), and to determine which of these markers best reflects the aging process in women. A total of 144 women aged 20-59 yr were enrolled in this cross-sectional study. Blood samples were obtained on cycle day 3 of regularly menstruating women (n=111), or at random in postmenopausal women (n=33). Data were analyzed with respect to premenopausal women age groups and compared in pre- and postmenopausal women. Area under the receiver operating characteristic curve (ROCAUC) analyses were performed to assess the ability of each marker to discriminate between the pre- and postmenopausal status. Serum levels of AMH, IGF-I, and IGFBP-3 decreased and serum levels of FSH increased significantly with age in premenopausal women. Serum luteinizing hormone (LH) was higher and inhibin B was lower in women in their 20-30's than in 40's. Serum levels of AMH and IGF-I showed a consistent decrease with all age groups. ROCAUC analysis showed that the diagnostic accuracy of AMH for menopausal status was similar to those of FSH, LH, and inhibin B, and was better than that of IGF-I. In conclusion, the serum AMH level appears to be the best marker of the aging process in premenopausal women.
Keyword
MeSH Terms
Figure
Reference
-
1. Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986. 233:1389–1394.
Article2. Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing, and early-growing follicles in aging women. Biol Reprod. 1994. 50:653–663.
Article3. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998. 69:505–510.4. Lenton EA, Sexton L, Lee S, Cooke ID. Progressive changes in LH and FSH and LH: FSH ratio in women throughout reproductive life. Maturitas. 1988. 10:35–43.
Article5. Lee SJ, Lenton EA, Sexton L, Cooke ID. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod. 1988. 3:851–855.
Article6. Rose MP, Gaines Das RE, Balen AH. Definition and measurement of follicle stimulating hormone. Endocr Rev. 2000. 21:5–22.7. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002. 77:357–362.8. Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002. 124:601–609.
Article9. Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999. 84:4025–4030.
Article10. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996. 17:121–155.
Article11. Woodruff TK, Lyon RJ, Hansen SE, Rice GC, Mather JP. Inhibin and activin locally regulate rat ovarian folliculogenesis. Endocrinology. 1990. 127:3196–3205.
Article12. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995. 16:3–34.
Article13. Clemmons DR, Dehoff ML, Busby WH, Bayne ML, Cascieri MA. Competition for binding to insulin-like growth factor (IGF) binding protein-2, 3, 4, and 5 by the IGFs and IGF analogs. Endocrinology. 1992. 131:890–895.
Article14. Blum WF, Ranke MB, Kietzmann K, Gauggel E, Zeisel HJ, Bierich JR. A specific radioimmunoassay for the growth hormone (GH)-dependent somatomedin-binding protein: its use for diagnosis of GH deficiency. J Clin Endocrinol Metab. 1990. 70:1292–1298.
Article15. Stadtmauer L, Vidali A, Lindheim SR, Sauer MV. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-binding protein-1 and -3 vary as a function of ovarian reserve and ovarian stimulation. J Assist Reprod Genet. 1998. 15:587–593.16. Klein NA, Battaglia DE, Miller PB, Soules MR. Circulating levels of growth hormone, insulin-like growth factor-I, and growth hormone binding protein in normal women of advanced reproductive age. Clin Endocrinol (Oxf). 1996. 44:285–292.
Article17. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988. 44:837–845.
Article18. Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996. 81:2742–2745.
Article19. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000. 21:200–214.
Article20. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen APN, te Velde ER. Serum antimüllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005. 83:979–987.
Article21. Schipper I, de Jong FH, Fauser BC. Lack of correlation between maximum early follicular phase serum follicle stimulating hormone concentrations and menstrual cycle characteristics in women under the age of 35 years. Hum Reprod. 1998. 13:1442–1448.
Article22. Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003. 18:323–327.23. Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981. 67:1361–1369.
Article24. Franchimont P, Urbain-Choffray D, Lambelin P, Fontaine MA, Frangin G, Reginster JY. Effects of repetitive administration of growth hormone-releasing hormone on growth hormone secretion, insulin-like growth factor I, and bone metabolism in postmenopausal women. Acta Endocrinol (Copenh). 1989. 120:121–128.
Article25. Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J Endocrinol. 1990. 126:R1–R4.
Article26. Mason HD, Margara R, Winston RM, Seppala M, Koistinen R, Franks S. Insulin-like growth factor-I (IGF-I) inhibits production of IGF-binding protein-1 while stimulating estradiol secretion in granulosa cells from normal and polycystic human ovaries. J Clin Endocrinol Metab. 1993. 76:1275–1279.
Article27. Di Blasio AM, Vigano P, Ferrari A. Insulin-like growth factor-II stimulates human granulosa-luteal cell proliferation in vitro. Fertil Steril. 1994. 61:483–487.
Article28. Yong EL, Baird DT, Yates R, Reichert LE Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992. 74:842–849.29. Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996. 137:1447–1456.
Article30. Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999. 84:105–111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship of Insulin like Growth Factor I with Pharmacologically Stimulated Growth Hormone Secretion in Growth Hormone Deficient Children
- Serum Insulin-like Growth Factors and their Binding Proteins in the Women With Polycystic Ovary
- Profile of Insulin, Growth Hormone and Insulin-like Growth Factors in Human Cord Blood According to Birth Weight
- Serum IGF-1 and IGFBP-3 Levels in Central Precocious Puberty Girls Treated with Gonadotropin Releasing Hormone Agonist (GnRHa)
- Serum levels of free insulin-like growth factor-I and clinical value in healthy children