J Korean Med Sci.
2008 Feb;23(1):10-17. 10.3346/jkms.2008.23.1.10.
Economic and Clinical Benefits of Galantamine in the Treatment of Mild to Moderate Alzheimer's Disease in a Korean Population: A 52-Week Prospective Study
- Affiliations
-
- 1Department of Psychiatry, Hallym University College of Medicine, Seoul, Korea. suhgh@chol.com
- 2Department of Psychiatry, Boramae Hospital, Seoul National University, College of Medicine, Seoul, Korea.
- 3Department of Psychiatry, the Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Janssen Korea Pharmaceutical, Seoul, Korea.
- KMID: 1786838
- DOI: http://doi.org/10.3346/jkms.2008.23.1.10
Abstract
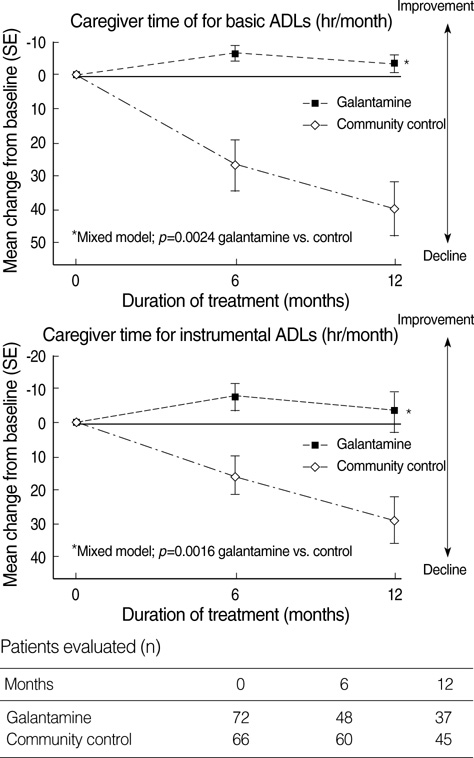
- To evaluate the impact of galantamine treatment on the function, caregiver time, and resource used in the treatment of patients with mild to moderate Alzheimer's disease (AD), costs and outcomes were evaluated during a 52-week prospective, randomized, double-blind, community-controlled trial of galantamine. Patients received either galantamine treatment (n=72) or no treatment (n=66). The analysis was performed from a societal perspective. Galantamine treatment reduced time spent caring for the patients and maintained improved functional capacity, whereas, when no treatments were given, a great increase in caregiver time and progressive functional deteriorations were observed. Saved caregiver time was equivalent to 113 working days per year. After 52 weeks, mean total annual costs per patient were 14,735,000 Korea Won (KRW) (USD 12,315) for patients with galantamine treatment and 25,325,000 KRW (USD 21,166) for patients without treatment. Adjusted annual cost saving of galantamine treatment was 6,428,000 KRW (USD 5,372) when compared to no treatment (p=0.0089). Galantamine had a beneficial effect not only to slow functional decline in patients with mild to moderate AD, but also to save a substantial amount of costs, closely related to reduction in caregiver burden and decrease in caregiver time.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
How Much Amount of Socioeconomic Loss Is Caused by Digestive Diseases?
Kyung Sik Park
Korean J Gastroenterol. 2011;58(6):297-299. doi: 10.4166/kjg.2011.58.6.297.
Reference
-
1. Suh GH, Kim JK, Cho MJ. Community study of dementia in the older Korean population. Aust N Z J Psychiatry. 2003. 37:606–612.2. Bores GM, Huger FP, Petko W, Mutlib AE, Camacho F, Rush DK, Selk DE, Wof V, Kosley RW Jr, Davis L, Vargas HM. Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galanthamine. J Pharmacol Exp Ther. 1996. 277:728–738.3. Chu LW, Yik PY, Mok W, Chung CP. A 2-year open-label study of galantamine therapy in Chinese Alzheimer's disease patients in Hong Kong. Int J Clin Pract. 2007. 61:403–410.
Article4. Pirttila T, Wilcock G, Truyen L, Damaraju CV. Long-term efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre trial. Eur J Neurol. 2004. 11:734–741.5. Suh GH, Jung HY, Lee CU, Oh BH, Bae JN, Jung HY, Ju YS, Yeon BK, Park J, Hong I, Choi S, Lee HJ. A prospective, double-blind, community-controlled comparison of three doses of galantamine in the treatment of mild to moderate Alzheimer's disease in a Korean population. Clin Ther. 2004. 26:1608–1618.
Article6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 1994. 4th ed. Washington, DC.: American Psychiatric Association.7. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984. 34:939–944.
Article8. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975. 12:189–198.9. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984. 141:1356–1364.10. Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther. 1999. 53:471–481.
Article11. Suh GH. Development of the Korean version of the Disability Assessment for Dementia Scale. J Korean Geriatr Soc. 2003. 7:278–287.12. Suh GH. Validation of the Korean version of the Alzheimer's Disease Assessment Scale. J Korean Geriatr Soc. 2003. 7:267–277.13. Reisberg B, Borenstein J, Salob S, Ferris S, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry. 1987. 48 :Suppl. 9–15.14. Suh GH, Son HG, Shin H, Kim IM, Hong S, Park J, Choi IG, Kim SK, Yeon BK. Reliability and analysis of symptom category scores of the behavior pathology in Alzheimer's disease rating scale, Korean version (BEHAVE-AD-K). J Korean Geriatr Psychiatry. 2001. 5:50–57.15. Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982. 139:1136–1139.16. Beecham J, Knapp M. Thornicroft G, editor. Costing psychiatric interventions. Measuring Mental Health Needs. 2001. 2nd edn. London: Gaskell;200–224.17. Wimo A, Mastey V, Winblad B. Wimo A, Jonsson B, Karlsson G, Winblad B, editors. Evaluation of the healthcare resource utilization and caregiver time in antidementia drug trials-a quantitative battery. Health economics of dementia. 1998. Chichester, UK: John Wiley & Sons;465–499.18. Hyoplus. WWW document. Accessed March 20, 2006. Available from: http://www.koreanursing.co.kr/service/service_gasa.php.19. Choi BH, Sunwoo D, Choi HM. Report on the introduction of public long-term care insurance for the elderly in Korea. 2001. Seoul: Korean Institute of Health and Social Affairs.20. National Health Insurance Corporation. Year 2002 National Health Insurance Statistical Yearbook. 2003. Seoul: National Health Insurance Corporation.21. Ministry of Health and Welfare. Guideline for health and welfare program for the Elderly. 2004. Seoul: Ministry of Health and Welfare.22. June KJ, Park JY. Cost-effectiveness analysis of home health care program for cerebrovascular accident patients. J Korean Community Nursing. 2001. 12:22–31.23. Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000. 54:2269–2276.24. Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantmine International-1 Study Group. BMJ. 2000. 321:1445–1449.25. Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer's disease: a randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2001. 71:589–595.
Article26. Wilkinson D, Murray J. Galantamine: a randomized, double-blind, dose comparison in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2001. 16:852–857.
Article27. Suh GH, Ju YS, Yeon BK, Shah A. A longitudinal study of Alzheimer's disease: rates of cognitive and functional decline. Int J Geriatr Psychiatry. 2004. 19:817–824.
Article28. Aneshensel CS, Pearlin LI, Levy-Storms L, Schuler RH. The transition from home to nursing home mortality among people with dementia. J Gerontol B Psychol Sci Soc Sci. 2000. 55:S152–S162.
Article29. Jönsson L, Jönsson B, Wimo A, Whitehouse P, Winblad B. Second international pharmacoeconomic conference on Alzheimer's disease. Alzheimer Dis Assoc Disord. 2000. 14:137–140.
Article30. Getsios D, Caro JJ, Caro G, Ishak K. Assessment of health economics in Alzheimer's disease (AHEAD) Galantamine treatment in Canada. Neurology. 2001. 57:972–978.
Article31. Caro JJ, Salas M, Ward A, Getsios D, Mehnert A. Economic analysis of galantamine, a cholinesterase inhibitor, in the treatment of patients with mild to moderate Alzheimer's disease in the Netherlands. Dement Geriatr Cogn Disord. 2002. 14:84–89.
Article32. Pocock SJ. The combination of randomized and historical controls in clinical trials. J Chronic Dis. 1976. 29:175–188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Galantamine on Caregiver Time and Activities of Daily Living in Mild to Moderate Alzheimer's Disease: A 1-Year Prospective Study
- A Study on the Association Between Cholineacetyltransferase(ChAT) Polymorphism and Treatment Responses of Galantamine in Alzheimer's Disease Patients
- Efficacy of Galantamine on Cognition in Mild-to-Moderate Alzheimer's Dementia after Failure to Respond to Donepezil
- The Effects of Galantamine Treatment on Attention and Its Relationship with Cognition and Activities of Daily Living in Patients with Mild to Moderate Alzheimer's Disease
- Effects of Galantamine Treatment on Attention, Activities of Daily Living, and Neuropsychiatric Symptoms between the Patients with Pure Alzheimer's Disease and Mixed Dementia