J Korean Med Sci.
2004 Jun;19(3):341-344. 10.3346/jkms.2004.19.3.341.
Rapid Prenatal Diagnosis of Down Syndrome Using Quantitative Fluorescent PCR in Uncultured Amniocytes
- Affiliations
-
- 1Laboratory of Medical Genetics, Samsung Cheil Hospital & Women's Healthcare Center, Sungkyunkwan University, School of Medicine, Seoul, Korea. genelab@samsung.co.kr
- 2Department of Obstetrics and Gynecology, Samsung Cheil Hospital & Women's Healthcare Center, Sungkyunkwan University, School of Medicine, Seoul, Korea.
- KMID: 1786809
- DOI: http://doi.org/10.3346/jkms.2004.19.3.341
Abstract
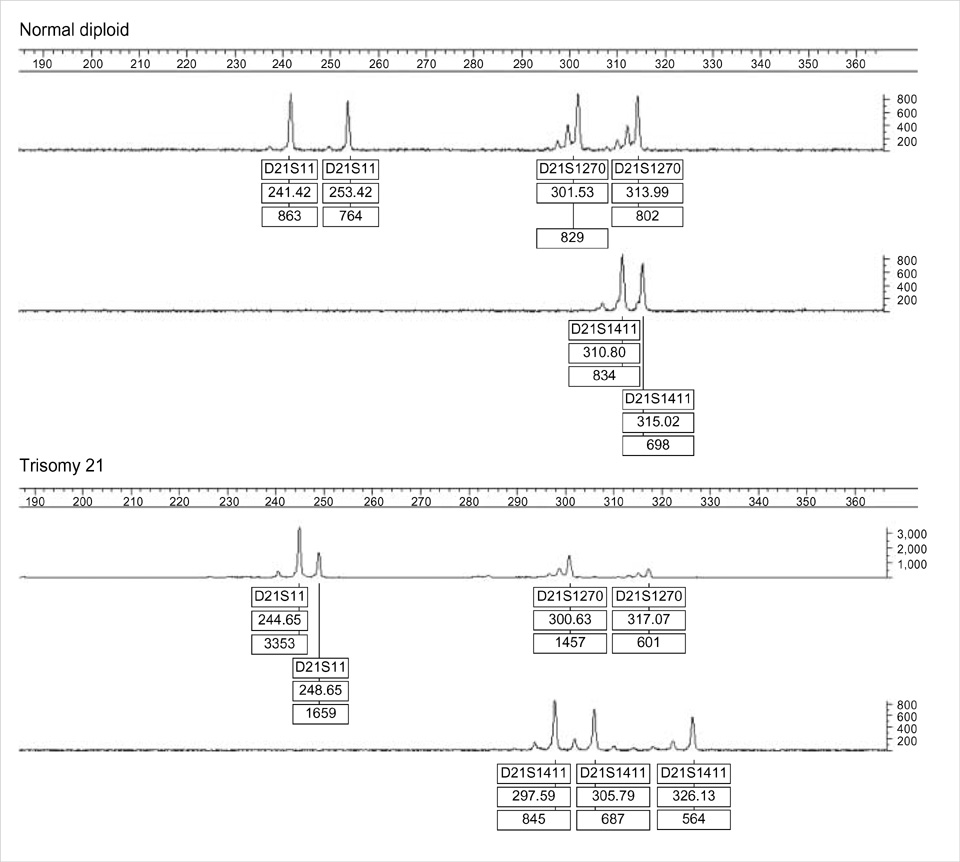
- Rapid prenatal diagnosis of common chromosome aneuploidies have been successful through quantitative fluoresent PCR (QF-PCR) assays and small tandem repeat (STR) markers. The purpose of our study was to investigate the clinical feasibility for rapid prenatal detection of Down syndrome using the quantitative fluorescent PCR in uncultured amniocytes. DNA was extracted from uncultured amniotic fluid of normal karyotype (n=200) and of Down syndrome (n=21). It was amplified using QF-PCR with four STR markers located on chromosome 21. Among normal samples, the ranges of diallelic peaks for at least one STR marker were 1.0-1.3 for D21S11, 1.0-1.4 for D21S1411 and 1.0-1.5 for D21S1270. Down syndrome samples showed trisomic triallelic patterns or trisomic diallelic patterns. The sensitivity, specificity, and efficiency of the assay for detecting Down syndrome were 95.4%, 100%, and 99.5%, respectively. Rapid prenatal diagnosis of Down syndrome using QF-PCR is a reliable technique that aids clinical management of pregnancy.
MeSH Terms
-
Alleles
Amniotic Fluid/*cytology
Chromosomes, Human, Pair 21
DNA/metabolism
Down Syndrome/*diagnosis
Female
Human
Korea
Microscopy, Fluorescence/*methods
Polymerase Chain Reaction/*methods
Polymorphism (Genetics)
Pregnancy
Prenatal Diagnosis/*methods
Sensitivity and Specificity
Support, Non-U.S. Gov't
Tandem Repeat Sequences
Time Factors
Figure
Reference
-
1. Klinger K, Landes G, Shook D, Harvey R, Lopez L, Locke P, Lerner T, Osathanondh R, Leverone B, Houseal T. Rapid detection of chromosome aneuploidies in uncultured amniocytes by using fluorescence in situ hybridization (FISH). Am J Hum Genet. 1992. 51:55–65.2. Thilaganathan B, Sairam S, Ballard T, Peterson C, Meredith R. Effectiveness of prenatal chromosomal analysis using multicolor fluorescent in situ hybridization. Bjog. 2000. 107:262–266.3. Mansfield ES. Diagnosis of Down syndrome and other aneuploidies using quantitative polymerase chain reaction and small tandem repeat polymorphisms. Hum Mol Genet. 1993. 2:43–50.
Article4. Pertl B, Yau SC, Sherlock J, Davies AF, Mathew CG, Adinolfi M. Rapid molecular method for prenatal detection of Down's syndrome. Lancet. 1994. 343:1197–1198.
Article5. Pertl B, Weitgasser U, Kopp S, Kroisel PM, Sherlock J, Adinolfi M. Rapid detection of trisomies 21 and 18 and sexing by quantitative fluorescent multiplex PCR. Hum Genet. 1996. 98:55–59.
Article6. Toth T, Findlay I, Papp C, Toth-Pal E, Marton T, Nagy B, Quirke P, Papp Z. Prenatal detection of trisomy 21 and 18 from amniotic fluid by quantitative fluorescent polymerase chain reaction. J Med Genet. 1998. 35:126–129.
Article7. Verma L, Macdonald F, Leedham P, McConachie M, Dhanjal S, Hulten M. Rapid and simple prenatal DNA diagnosis of Down's syndrome. Lancet. 1998. 352:9–12.
Article8. Schmidt W, Jenderny J, Hecher K, Hackeloer BJ, Kerber S, Kochhan L, Held KR. Detection of aneuploidy in chromosomes X, Y, 13, 18 and 21 by QF-PCR in 662 selected pregnancies at risk. Mol Hum Reprod. 2000. 6:855–860.
Article9. Levett LJ, Liddle S, Meredith R. A large-scale evaluation of amnio-PCR for the rapid prenatal diagnosis of fetal trisomy. Ultrasound Obstet Gynecol. 2001. 17:115–118.
Article10. Mann K, Fox SP, Abbs SJ, Yau SC, Scriven PN, Docherty Z, Ogilvie CM. Development and implementation of a new rapid aneuploidy diagnostic service within the UK National Health Service and implications for the future of prenatal diagnosis. Lancet. 2001. 358:1057–1061.
Article11. Pertl B, Kopp S, Kroisel PM, Hausler M, Sherlock J, Winter R, AdinolfiM . Quantitative fluorescence polymerase chain reaction for the rapid prenatal detection of common aneuploidies and fetal sex. Am J Obstet Gynecol. 1997. 177:899–906.
Article12. Ferre F. Quantitative or semi-quantitative PCR: reality versus myth. PCR Methods Appl. 1992. 2:1–9.
Article13. Han GR, Lee YW, Lee HL, Kim SM, Ku TW, Kang IH, Lee HS, Hwang JJ. A Korean population study of the nine STR loci FGA, VWA, D3S1358, D18S51, D21S11, D8S1179, D7S820, D13S317 and D5S818. Int J Legal Med. 2000. 114:41–44.
Article14. Lee JW, Lee HS, Hwang JJ. Statistical analysis for estimating heterogeneity of the Korean population in DNA typing using STR loci. Int J Legal Med. 2002. 116:153–160.
Article15. Kim YL, Hwang JY, Kim YJ, Lee S, Chung NG, Goh HG, Kim CC, Kim DW. Allele frequencies of 15 STR loci using AmpF/STR Identifiler kit in a Korean population. Forensic Sci Int. 2003. 136:92–95.
Article16. Yoon HR, Park YS, Kim YK. Rapid prenatal detection of Down and Edwards syndromes by fluorescent polymerase chain reaction with short tandem repeat markers. Yonsei Med J. 2002. 43:557–566.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid detection of aneuploidy using FISH in uncultured amniocytes for prenatal diagnosis : 8-year experience
- Prenatal Aneuploidy Detection in Uncultured Amniotic Fluid Interphase Cells by Fluorescence in situ Hybridization (FISH)
- Rapid Diagnosis of Duchenne Muscular Dystrophy DMD by Multiplex Polymerase Chain Reaction PCR using Uncultured Amniocytes
- Detection of Down Syndrome & Edward Syndrome in uncultured amniocytes using FISH ( Fluorescence In Situ Hybridization
- Rapid prenatal diagnosis of spinocerebellar ataxia type 3 by using fluorescent PCR