J Korean Med Sci.
2011 Oct;26(10):1344-1355. 10.3346/jkms.2011.26.10.1344.
Electrode Position and the Clinical Outcome after Bilateral Subthalamic Nucleus Stimulation
- Affiliations
-
- 1Movement Disorder Center and Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. brain@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- 3Department of Neurology, Seoul National University Hospital, Seoul, Korea.
- 4Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 5Neuroscience Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 6Ischemia Hypoxia Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- 7Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 83D Medical Imaging Lab, CyberMed, Seoul, Korea.
- KMID: 1785994
- DOI: http://doi.org/10.3346/jkms.2011.26.10.1344
Abstract
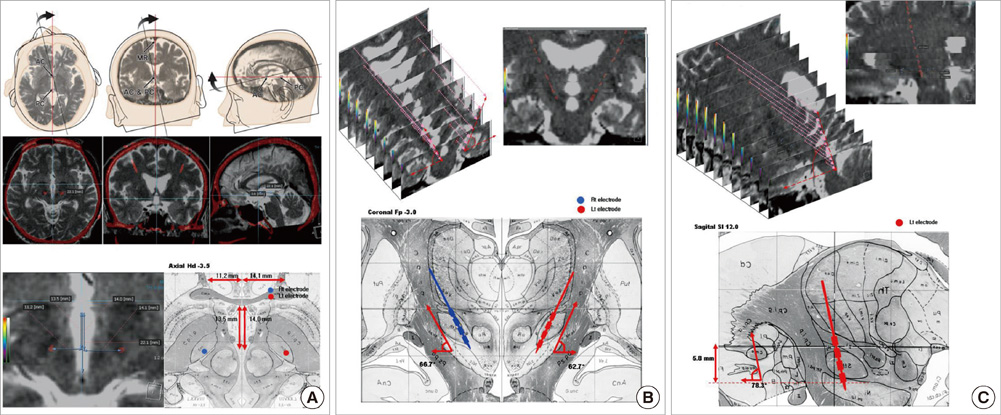
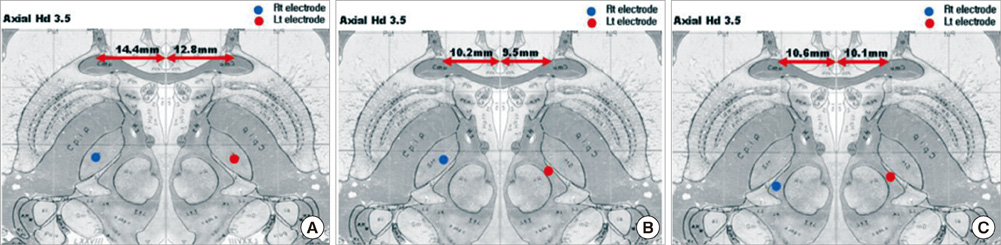
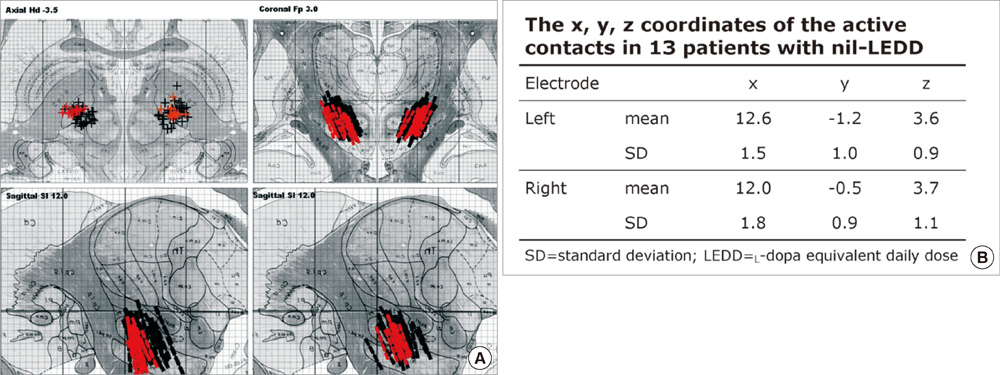
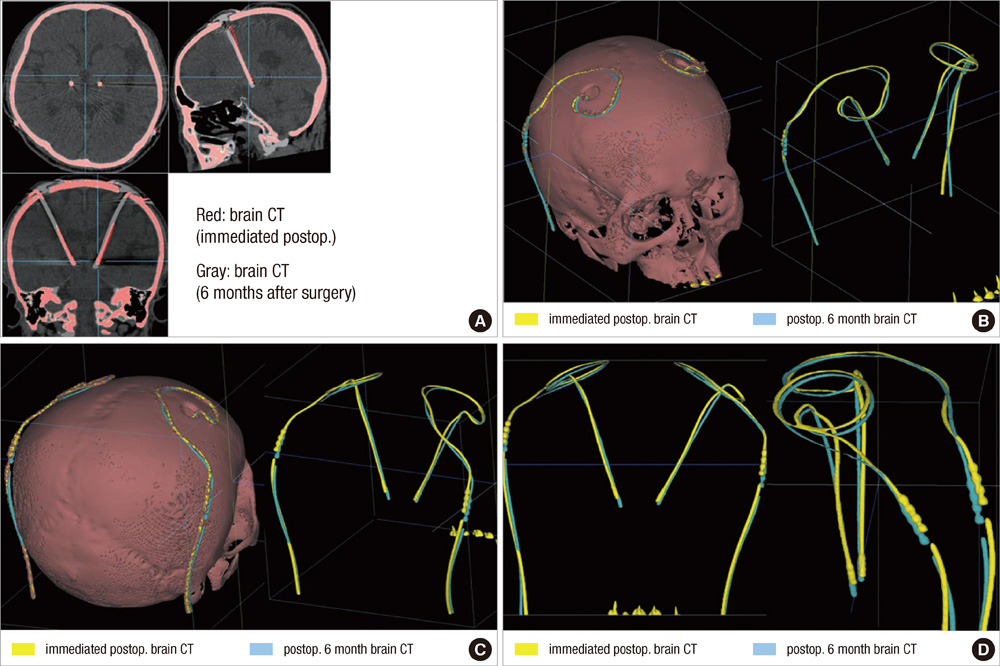
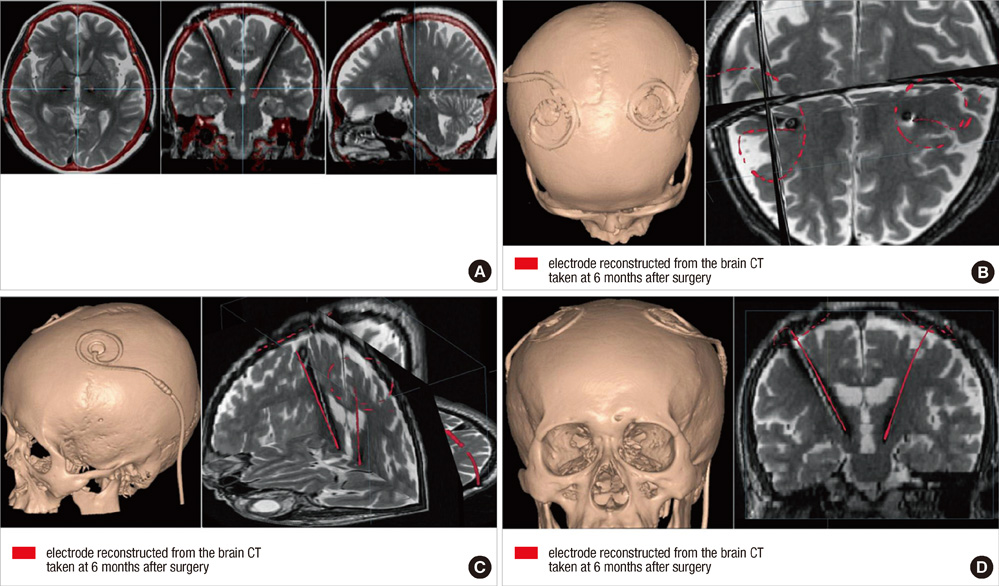
- We compared the surgical outcome with electrode positions after bilateral subthalamic nucleus (STN) stimulation surgery for Parkinson's disease. Fifty-seven patients treated with bilateral STN stimulations were included in this study. Electrode positions were determined in the fused images of preoperative MRI and postoperative CT taken at six months after surgery. The patients were divided into three groups: group I, both electrodes in the STN; group II, only one electrode in the STN; group III, neither electrode in the STN. Unified Parkinson's Disease Rating Scale (UPDRS), Hoehn and Yahr stage, and activities of daily living scores significantly improved at 6 and 12 months after STN stimulation in both group I and II. The off-time UPDRS III speech subscore significantly improved (1.6 +/- 0.7 at baseline vs 1.3 +/- 0.8 at 6 and 12 months, P < 0.01) with least L-dopa equivalent daily dose (LEDD) (844.6 +/- 364.1 mg/day at baseline; 279.4 +/- 274.6 mg/day at 6 months; and 276.0 +/- 301.6 mg/day at 12 months, P < 0.001) at 6 and 12 months after STN deep brain stimulation (DBS) in the group I. Our findings suggest that the better symptom relief including speech with a reduced LEDD is expected in the patients whose electrodes are accurately positioned in both STN.
Keyword
MeSH Terms
-
Adult
Aged
Antiparkinson Agents/adverse effects/*therapeutic use
Combined Modality Therapy
*Deep Brain Stimulation/adverse effects/instrumentation/methods
*Electrodes, Implanted
Female
Humans
Levodopa/adverse effects/therapeutic use
Magnetic Resonance Imaging
Male
Middle Aged
Parkinson Disease/drug therapy/*therapy
Severity of Illness Index
Subthalamic Nucleus/*physiology
Treatment Outcome
Figure
Cited by 1 articles
-
Factors Related to Outcomes of Subthalamic Deep Brain Stimulation in Parkinson's Disease
Hae Yu Kim, Won Seok Chang, Dong Wan Kang, Young Ho Sohn, Myung Sik Lee, Jin Woo Chang
J Korean Neurosurg Soc. 2013;54(2):118-124. doi: 10.3340/jkns.2013.54.2.118.
Reference
-
1. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003. 349:1925–1934.2. Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998. 339:1105–1111.3. Bejjani BP, Dormont D, Pidoux B, Yelnik J, Damier P, Arnulf I, Bonnet AM, Marsault C, Agid Y, Philippon J, Cornu P. Bilateral subthalamic stimulation for Parkinson's disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg. 2000. 92:615–625.4. Godinho F, Thobois S, Magnin M, Guenot M, Polo G, Benatru I, Xie J, Salvetti A, Garcia-Larrea L, Broussolle E, Mertens P. Subthalamic nucleus stimulation in Parkinson's disease: anatomical and electrophysiological localization of active contacts. J Neurol. 2006. 253:1347–1355.5. Lanotte MM, Rizzone M, Bergamasco B, Faccani G, Melcarne A, Lopiano L. Deep brain stimulation of the subthalamic nucleus: anatomical and neurophysiological and outcome correlations with the effects of stimulation. J Neurol Neurosurg Psychiatry. 2002. 72:53–58.6. Saint-Cyr JA, Hoque T, Pereira LC, Dostrovsky JO, Hutchison WD, Mikulis DJ, Abosch A, Sime E, Lang AE, Lozano AM. Localization of clinically effective stimulating electrodes in the human subthalamic nucleus on magnetic resonance imaging. J Neurosurg. 2002. 97:1152–1166.7. Yelnik J, Damier P, Demeret S, Gervais D, Bardinet E, Bejjani BP, François C, Houeto JL, Arnule I, Dormont D, Galanaud D, Pidoux B, Cornu P, Agid Y. Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method. J Neurosurg. 2003. 99:89–99.8. Halpern CH, Danish SF, Baltuch GH, Jaggi JL. Brain shift during deep brain stimulation surgery for Parkinson's disease. Stereotact Funct Neurosurg. 2008. 86:37–43.9. Khan MF, Mewes K, Gross RE, Skrinjar O. Assessment of brain shift related to deep brain stimulation surgery. Stereotact Funct Neurosurg. 2008. 86:44–53.10. Miyagi Y, Shima F, Sasaki T. Brain shift: an error factor during implantation of deep brain stimulation electrodes. J Neurosurg. 2007. 107:989–997.11. Martinez-Santiesteban FM, Swanson SD, Noll DC, Anderson DJ. Magnetic field perturbation of neural recording and stimulating microelectrodes. Phys Med Biol. 2007. 52:2073–2088.12. Paek SH, Han JH, Lee JY, Kim C, Jeon BS, Kim DG. Electrode position determined by fused images of preoperative and postoperative magnetic resonance imaging and surgical outcome after subthalamic nucleus deep brain stimulation. Neurosurgery. 2008. 63:925–936.13. Heo JH, Lee KM, Paek SH, Kim MJ, Lee JY, Kim JY, Cho SY, Lim YH, Kim MR, Jeong SY, Jeon BS. The effects of bilateral subthalamic nucleus deep brain stimulation (STN DBS) on cognition in Parkinson disease. J Neurol Sci. 2008. 273:19–24.14. Christensen GE, Joshi SC, Miller MI. Volumetric transformation of brain anatomy. IEEE Trans Med Imaging. 1997. 16:864–877.15. Ferroli P, Franzini A, Marras C, Maccagnano E, D'Incerti L, Broggi G. A simple method to assess accuracy of deep brain stimulation electrode placement: pre-operative stereotactic CT+ postoperative MR image fusion. Stereotact Funct Neurosurg. 2004. 82:14–19.16. Ken S, Di Gennaro G, Giulietti G, Sebastiano F, De Carli D, Garreffa G, Colonnese C, Passariello R, Lotterie JA, Maraviglia B. Quantitative evaluation for brain CT/MRI coregistration based on maximization of mutual information in patients with focal epilepsy investigated with subdural electrodes. Magn Reson Imaging. 2007. 25:883–888.17. Kim HJ, Paek SH, Kim JY, Lee JY, Lim YH, Kim DG, Jeon BS. Two-year follow-up on the effect of unilateral subthalamic deep brain stimulation in highly asymmetric Parkinson's disease. Mov Disord. 2009. 24:329–335.18. Pluim JP, Maintz JB, Viergever MA. Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging. 2003. 22:986–1004.19. Schaltenbrand G, Wahren W. Atlas for stereotaxy of the human brain. 1977. 2nd ed. Stuttgart·New York: Thieme.20. Herzog J, Volkmann J, Krack P, Kopper F, Pötter M, Lorenz D, Steinbach M, Klebe S, Hamel W, Schrader B, Weinert D, Müller D, Mehdorn HM, Deuschl G. Two-year follow-up of subthalamic deep brain stimulation in Parkinson's disease. Mov Disord. 2003. 18:1332–1337.21. Coyne T, Silburn P, Cook R, Silberstein P, Mellick G, Sinclair F, Fracchia G, Wasson D, Stanwell P. Rapid subthalamic nucleus deep brain stimulation lead placement utilising CT/MRI fusion, microelectrode recording and test stimulation. Acta Neurochir Suppl. 2006. 99:49–50.22. Duffner F, Schiffbauer H, Breit S, Friese S, Freudenstein D. Relevance of image fusion for target point determination in functional neurosurgery. Acta Neurochir (Wien). 2002. 144:445–451.23. Hamel W, Fietzek U, Morsnowski A, Schrader B, Herzog J, Weinert D, Pfister G, Müller D, Volkmann J, Deuschl G, Mehdorn HM. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: evaluation of active electrode contacts. J Neurol Neurosurg Psychiatry. 2003. 74:1036–1046.24. Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthatlamic nucleus in improving contralateral parkinsonism. Brain. 2006. 129:1732–1747.25. Voges J, Volkmann J, Allert N, Lehrke R, Koulousakis A, Freund HJ, Sturm V. Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: correlation of therapeutic effect with anatomical electrode position. J Neurosurg. 2002. 96:269–279.26. McClelland S 3rd, Ford B, Senatus PB, Winfield LM, Du YE, Pullman SL, Yu Q, Frucht SJ, McKhann GM 2nd, Goodman RR. Subthalamic stimulation for Parkinson disease: determination of electrode location necessary for clinical efficacy. Neurosurg Focus. 2005. 19:E12.27. Kim YH, Kim HJ, Kim C, Kim DG, Jeon BS, Paek SH. Comparison of electrode location between immediate postoperative day and 6 months after bilateral subthalamic nucleus deep brain stimulation. Acta Neurochir (Wien). 2010. 152:2037–2045.28. Lee JY, Kim JW, Lee JY, Lim YH, Kim C, Kim DG, Jeon BS, Paek SH. Is MRI a reliable tool to locate the electrode after deep brain stimulation surgery? Comparison study of CT and MRI for the localization of electrodes after DBS. Acta Neurochir (Wien). 2010. 152:2029–2036.29. Benabid AL, Chabardès S, Seigneuret E. Deep brain stimulation in Parkinson's disease: long-term efficacy and safety - What happened this year? Curr Opin Neurol. 2005. 18:623–630.30. Pinto S, Thobois S, Costes N, Le Bars D, Benabid AL, Broussolle E, Pollak P, Gentil M. Subthalamic nucleus stimulation and dysarthria in Parkinson's disease: a PET study. Brain. 2004. 127:602–615.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Turning on the Left Side Electrode Changed Depressive State to Manic State in a Parkinson's Disease Patient Who Received Bilateral Subthalamic Nucleus Deep Brain Stimulation: A Case Report
- Deep Brain Stimulation of the Subthalamic and Pedunculopontine Nucleus in a Patient with Parkinson's Disease
- Microelectrode Recording-Guided Deep Brain Stimulation in Patients with Movement Disorders
- The Effect of Bilateral Subthalamic Nucleus Stimulation in Parkinson's Disease: Experiences From 6 Month Follow-up in Sinchon Severance Hospital
- Pilot Study for Considering Subthalamic Nucleus Anatomy during Stimulation Using Directional Leads