J Korean Med Sci.
2011 Jun;26(6):814-823. 10.3346/jkms.2011.26.6.814.
Spinal Cord Injury Markedly Altered Protein Expression Patterns in the Affected Rat Urinary Bladder during Healing Stages
- Affiliations
-
- 1MRCND and Department of Biochemistry, BK21 Program for Biomedical Sciences, School of Medicine and Institute of Health Sciences, Gyeongsang National University, Jinju, Korea. kkang@gnu.ac.kr
- 2MRCND and Department of Psychiatry, BK21 Program for Biomedical Sciences, School of Medicine and Institute of Health Sciences, Gyeongsang National University, Jinju, Korea.
- 3MRCND and Department of Physiology, BK21 Program for Biomedical Sciences, School of Medicine and Institute of Health Sciences, Gyeongsang National University, Jinju, Korea.
- 4MRCND and Department of Urology, BK21 Program for Biomedical Sciences, School of Medicine and Institute of Health Sciences, Gyeongsang National University, Jinju, Korea.
- 5Department of Physical Medicine and Rehabilitation, Changwon Samsung Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- KMID: 1785970
- DOI: http://doi.org/10.3346/jkms.2011.26.6.814
Abstract
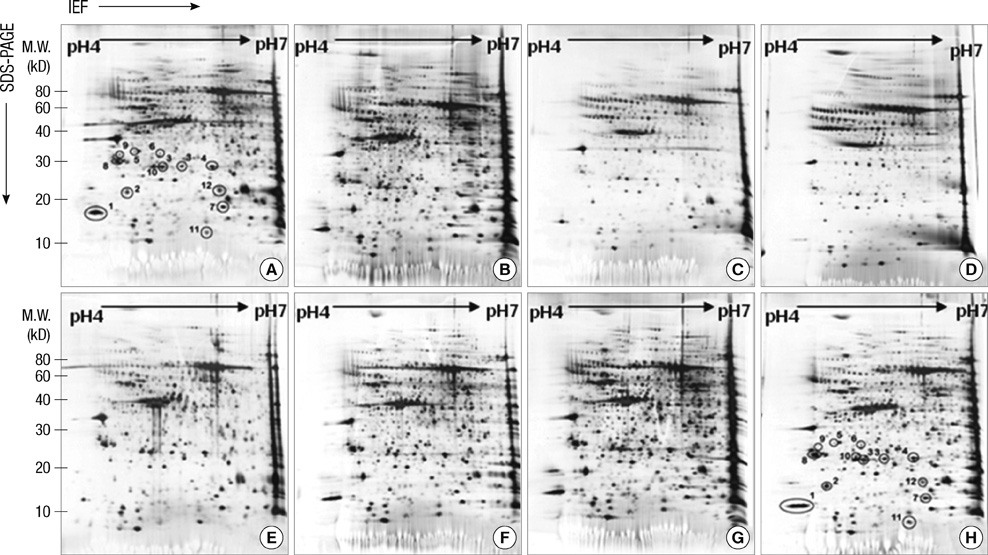
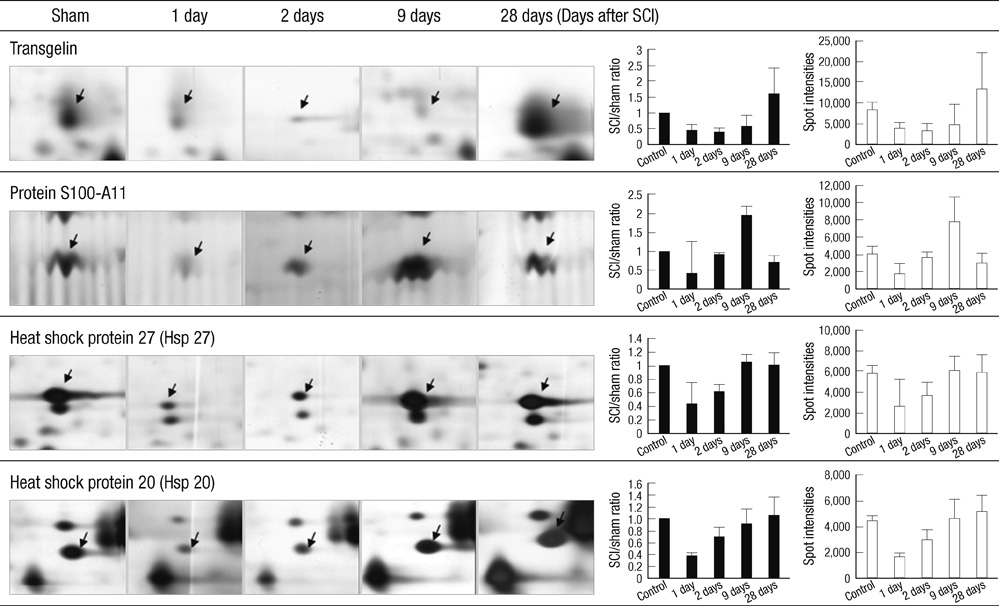
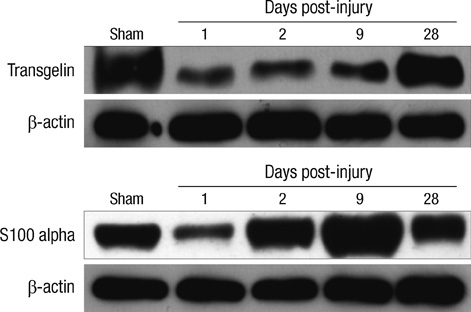
- The influence of spinal cord injury (SCI) on protein expression in the rat urinary bladder was assessed by proteomic analysis at different time intervals post-injury. After contusion SCI between T9 and T10, bladder tissues were processed by 2-DE and MALDI-TOF/MS at 6 hr to 28 days after SCI to identify proteins involved in the healing process of SCI-induced neurogenic bladder. Approximately 1,000 spots from the bladder of SCI and sham groups were visualized and identified. At one day after SCI, the expression levels of three protein were increased, and seven spots were down-regulated, including heat shock protein 27 (Hsp27) and heat shock protein 20 (Hsp20). Fifteen spots such as S100-A11 were differentially expressed seven days post-injury, and seven proteins including transgelin had altered expression patterns 28 days after injury. Of the proteins with altered expression levels, transgelin, S100-A11, Hsp27 and Hsp20 were continuously and variably expressed throughout the entire post-SCI recovery of the bladder. The identified proteins at each time point belong to eight functional categories. The altered expression patterns identified by 2-DE of transgelin and S100-A11 were verified by Western blot. Transgelin and protein S100-A11 may be candidates for protein biomarkers in the bladder healing process after SCI.
Keyword
MeSH Terms
-
Animals
Biological Markers/metabolism
Electrophoresis, Gel, Two-Dimensional
Female
HSP20 Heat-Shock Proteins/metabolism
HSP27 Heat-Shock Proteins/metabolism
Microfilament Proteins/metabolism
Muscle Proteins/metabolism
Proteome/*biosynthesis
Proteomics
Rats
Rats, Sprague-Dawley
S100 Proteins/metabolism
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Spinal Cord Injuries/*metabolism/pathology
Urinary Bladder/*metabolism
*Wound Healing
Figure
Reference
-
1. Saganová K, Orendácová J, Sulla I Jr, Filipcík P, Cízková D, Vanický I. Effects of long-term FK506 administration on functional and histopathological outcome after spinal cord injury in adult rat. Cell Mol Neurobiol. 2009. 29:1045–1051.2. Nath M, Wheeler JS Jr, Walter JS. Urologic aspects of traumatic central cord syndrome. J Am Paraplegia Soc. 1993. 16:160–164.3. Shen LF, Cheng H, Tsai MC, Kuo HS, Chak KF. PAL31 may play an important role as inflammatory modulator in the repair process of the spinal cord injury rat. J Neurochem. 2009. 108:1187–1197.4. Komori N, Takemori N, Kim HK, Singh A, Hwang SH, Foreman RD, Chung K, Chung JM, Matsumoto H. Proteomics study of neuropathic and nonneuropathic dorsal root ganglia: altered protein regulation following segmental spinal nerve ligation injury. Physiol Genomics. 2007. 29:215–230.5. Kang SK, So HH, Moon YS, Kim CH. Proteomic analysis of injured spinal cord tissue proteins using 2-DE and MALDI-TOF MS. Proteomics. 2006. 6:2797–2812.6. de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010. 29:63–76.7. Wognum S, Lagoa CE, Nagatomi J, Sacks MS, Vodovotz Y. An exploratory pathways analysis of temporal changes induced by spinal cord injury in the rat bladder wall: insights on remodeling and inflammation. PLoS One. 2009. 4:e5852.8. Jang I, La JH, Kim GT, Lee JS, Kim EJ, Lee ES, Kim SJ, Seo JM, Ahn SH, Park JY, Hong SG, Kang D, Han J. Single-channel recording of TASK-3-like K channel and up-regulation of TASK-3 mRNA expression after spinal cord injury in rat dorsal root ganglion neurons. Korean J Physiol Pharmacol. 2008. 12:245–251.9. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.10. Hwa JS, Kim HJ, Goo BM, Park HJ, Kim CW, Chung KH, Park HC, Chang SH, Kim YW, Kim DR, Cho GJ, Choi WS, Kang KR. The expression of ketohexokinase is diminished in human clear cell type of renal cell carcinoma. Proteomics. 2006. 6:1077–1084.11. Landry F, Lombardo CR, Smith JW. A method for application of samples to matrix-assisted laser desorption ionization time-of-flight targets that enhances peptide detection. Anal Biochem. 2000. 279:1–8.12. Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2004. 2:CD004375.13. Hansen J, Media S, Nøhr M, Biering-Sørensen F, Sinkjaer T, Rijkhoff NJ. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol. 2005. 173:2035–2039.14. Mitsui T, Fischer I, Shumsky JS, Murray M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol. 2005. 194:410–431.15. Yan X, Liu J, Luo Z, Ding Q, Mao X, Yan M, Yang S, Hu X, Huang J, Luo Z. Proteomic profiling of proteins in rat spinal cord induced by contusion injury. Neurochem Int. 2010. 56:971–983.16. Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol. 2007. 579:877–892.17. van Hemert MJ, Steensma HY, van Heusden GP. 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays. 2001. 23:936–946.18. Dreiza CM, Brophy CM, Komalavilas P, Furnish EJ, Joshi L, Pallero MA, Murphy-Ullrich JE, von Rechenberg M, Ho YS, Richardson B, Xu N, Zhen Y, Peltier JM, Panitch A. Transducible heat shock protein 20 (HSP20) phosphopeptide alters cytoskeletal dynamics. FASEB J. 2005. 19:261–263.19. Chiavegato A, Roelofs M, Franch R, Castellucci E, Sarinella F, Sartore S. Differential expression of SM22 isoforms in myofibroblasts and smooth muscle cells from rabbit bladder. J Muscle Res Cell Motil. 1999. 20:133–146.20. Prasad PD, Stanton JA, Assinder SJ. Expression of the actin-associated protein transgelin (SM22) is decreased in prostate cancer. Cell Tissue Res. 2010. 339:337–347.21. Han M, Dong LH, Zheng B, Shi JH, Wen JK, Cheng Y. Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci. 2009. 84:394–401.22. Tyagi P, Chen X, Hayashi Y, Yoshimura N, Chancellor MB, de Miguel F. Proteomic investigation on chronic bladder irritation in the rat. Urology. 2008. 71:536–540.23. Kim HJ, Sohng I, Kim DH, Lee DC, Hwang CH, Park JY, Ryu JW. Investigation of early protein changes in the urinary bladder following partial bladder outlet obstruction by proteomic approach. J Korean Med Sci. 2005. 20:1000–1005.24. Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995. 37:417–429.25. Naka M, Qing ZX, Sasaki T, Kise H, Tawara I, Hamaguchi S, Tanaka T. Purification and characterization of a novel calcium-binding protein, S100C, from porcine heart. Biochim Biophys Acta. 1994. 1223:348–353.26. Kanamori T, Takakura K, Mandai M, Kariya M, Fukuhara K, Sakaguchi M, Huh NH, Saito K, Sakurai T, Fujita J, Fujii S. Increased expression of calcium-binding protein S100 in human uterine smooth muscle tumours. Mol Hum Reprod. 2004. 10:735–742.27. Dupont-Versteegden EE, Houlé JD, Dennis RA, Zhang J, Knox M, Wagoner G, Peterson CA. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004. 29:73–81.28. Batts TW, Klausner AP, Jin Z, Meeks MK, Ripley ML, Yang SK, Tuttle JB, Steers WD, Rembold CM. Increased expression of heat shock protein 20 and decreased contractile stress in obstructed rat bladder. J Urol. 2006. 176:1679–1684.29. Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacol Ther. 2008. 119:44–54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in the Expression of Smooth Muscle Myosin Heavy Chain mRNA following Partial Bladder Obstruction or Spinal Cord Injury in Rat: A Preliminary Study
- Spinal Cord Injury Rehabilitation (II): Management of Neurogenic Bladder
- Effect of Terazosin on Low Compliant Bladder in Patients with Spinal Cord Injury
- A Clinical Observation on Urinary Calculi in Traumatic Spinal Cord Injured Patient
- The problems of bladder overdistention in patients with spinal cord injury