J Korean Med Sci.
2011 Jun;26(6):759-764. 10.3346/jkms.2011.26.6.759.
Carcinoembryonic Antigen Level Can be Overestimated in Metabolic Syndrome
- Affiliations
-
- 1Department of Family Practice and Community Health, Ajou University School of Medicine, Suwon, Korea. djleemd@msn.com
- 2Department of Medical Informatics, Ajou University School of Medicine, Suwon, Korea.
- KMID: 1785961
- DOI: http://doi.org/10.3346/jkms.2011.26.6.759
Abstract
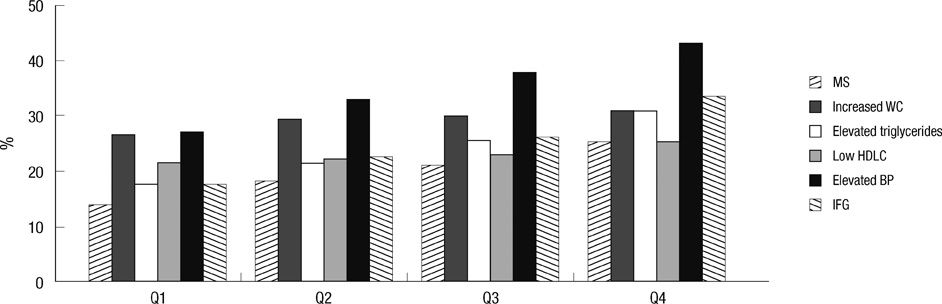
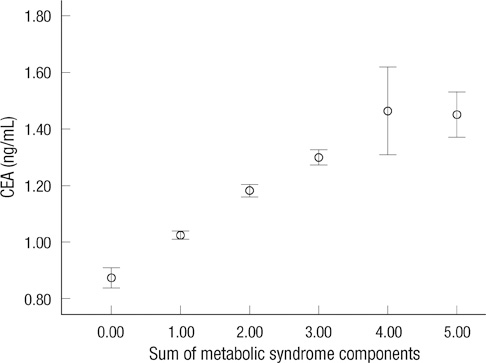
- Carcinoembryonic antigen (CEA) levels can be affected by many factors and metabolic syndrome is also a candidate. This study examined the relationship between CEA levels and metabolic syndrome using the data of 32,897 healthy Koreans. Fecal occult blood tests were also performed. Subjects with colorectal carcinoma were excluded. Subjects were classified by their smoking status, metabolic syndrome and its components. Prevalence of metabolic syndrome and its all components showed a significant increase according to the quartile of serum CEA concentration (P < 0.001). Increased numbers of metabolic syndrome components showed a positive association with CEA levels (P-trend < 0.001). The odds ratios for the highest CEA quartile vs the lowest serum CEA quartile significantly increased in the presence of metabolic syndrome and its components. After adjusting for age, gender and smoking status, metabolic syndrome, low high density lipoprotein cholesterol and elevated blood pressure had higher odds ratios (OR) of the highest CEA quartile compared with the lowest serum CEA quartile (OR = 1.125, 95% CI = 1.030 to 1.222, P = 0.009; OR = 1.296, 95% CI = 1.195 to 1.405, P < 0.001; OR = 1.334, 95% CI = 1.229 to 1.448, P < 0.001, respectively). These results indicate that metabolic syndrome is associated with CEA value, which may lead to a misunderstanding of the CEA levels.
MeSH Terms
Figure
Reference
-
1. Fukuda I, Yamakado M, Kiyose H. Influence of smoking on serum carcinoembryonic antigen levels in subjects who underwent multiphasic health testing and services. J Med Syst. 1998. 22:89–93.2. Amino N, Kuro R, Yabu Y, Takai SI, Kawashima M, Morimoto S, Ichihara K, Miyai K, Kumahara Y. Elevated levels of circulating carcinoembryonic antigen in hypothyroidism. J Clin Endocrinol Metab. 1981. 52:457–462.3. Bulut I, Arbak P, Coskun A, Balbay O, Annakkaya AN, Yavuz O, Gülcan E. Comparison of serum CA 19.9, CA 125 and CEA levels with severity of chronic obstructive pulmonary disease. Med Princ Pract. 2009. 18:289–293.4. Witherspoon LR, Shuler SE, Alyea K, Husserl FE. Carcinoembryonic antigen: assay following heat compared with perchloric acid extraction in patients with colon cancer, non-neoplastic gastrointestinal diseases, or chronic renal failure. J Nucl Med. 1983. 24:916–921.5. Herbeth B, Bagrel A. A study of factors influencing plasma CEA levels in an unselected population. Oncodev Biol Med. 1980. 1:191–198.6. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.7. Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002. 156:1070–1077.8. Furberg AS, Veierød MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004. 96:1152–1160.9. Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002. 11:385–391.10. Michaud DS, Liu S, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study. J Natl Cancer Inst. 2002. 94:1293–1300.11. Park JS, Choi GS, Jang YS, Jun SH, Kang H. Influence of obesity on the serum carcinoembryonic antigen value in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010. 19:2461–2468.12. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. American Heart Association. National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005. 112:2735–2752.13. Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007. 75:72–80.14. Kim MH, Kim MK, Choi BY, Shin YJ. Prevalence of the metabolic syndrome and its association with cardiovascular diseases in Korea. J Korean Med Sci. 2004. 19:195–201.15. Ryu SY, Kweon SS, Park HC, Shin JH, Rhee JA. Obesity and the metabolic syndrome in Korean adolescents. J Korean Med Sci. 2007. 22:513–517.16. Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, Adam HO. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control. 2001. 12:13–21.17. Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999. 91:1147–1154.18. Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006. 107:28–36.19. Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F. Risk Factors and Life Expectancy Research Group. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. 2001. 10:937–941.20. Ishizaka N, Ishizaka Y, Toda E, Koike K, Yamakado M, Nagai R. Are serum carcinoembryonic antigen levels associated with carotid atherosclerosis in Japanese men? Arterioscler Thromb Vasc Biol. 2008. 28:160–165.21. Vassalle C, Pratali L, Ndreu R, Battaglia D, Andreassi MG. Carcinoembryonic antigen concentrations in patients with acute coronary syndrome. Clin Chem Lab Med. 2010. 48:1339–1343.22. Zhang L, Li SN, Wang XN. CEA and AFP expression in human hepatoma cells transfected with antisense IGF-I gene. World J Gastroenterol. 1998. 4:30–32.23. Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006. 169:1505–1522.24. Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006. 12:1623–1635.25. Cea-Calvo L, Lozano JV, Fernández-Pérez C, Llisterri JL, Martí-Canales JC, Aznar J, Gil-Guillén V, Redón J. Investigators of PREV-ICTUS study. Prevalence of low HDL cholesterol, and relationship between serum HDL and cardiovascular disease in elderly Spanish population: the PREV-ICTUS study. Int J Clin Pract. 2009. 63:71–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Individualized Cutoff Value of the Serum Carcinoembryonic Antigen Level According to TNM Stage in Colorectal Cancer
- Long-Term Use of Escitalopram and a High Level of Carcinoembryonic Antigen
- Clinical Significance of Serum Carcinoembryonic Antigen Level in Rectal Cancer Patients Who Underwent Preoperative Chemoradiotherapy
- Assessment of prognostic role od serum carcinoembryonic antigen monitoring in patients with stomach, colorectal and breast cancers
- Serum Carcinoembryonic Antigen for Recurrence in Colorectal Cancer Patients