J Korean Med Sci.
2007 Dec;22(6):1022-1025. 10.3346/jkms.2007.22.6.1022.
Plasmodium falciparum Cultivation Using the Petri Dish: Revisiting the Effect of the 'Age' of Erythrocytes and the Interval of Medium Change
- Affiliations
-
- 1Department of Microbiology, Gachon Medical School, Incheon, Korea. seorak@dreamwiz.com
- 2Department of Occupational Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Microbiology, College of Medicine, Ewha Womans University, Seoul, Korea.
- KMID: 1785764
- DOI: http://doi.org/10.3346/jkms.2007.22.6.1022
Abstract
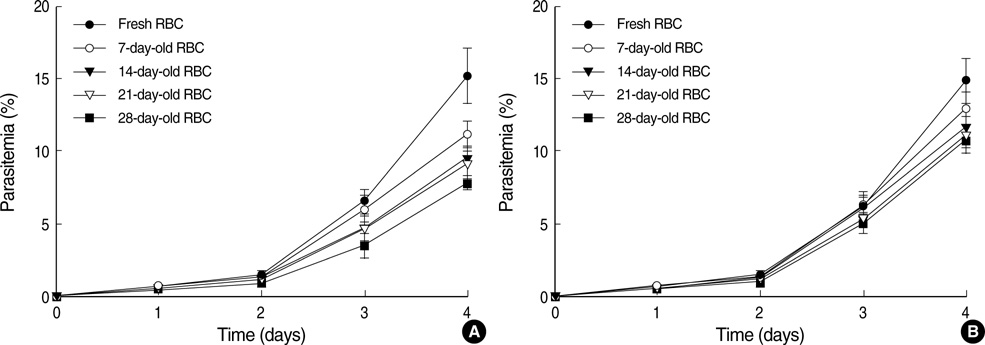
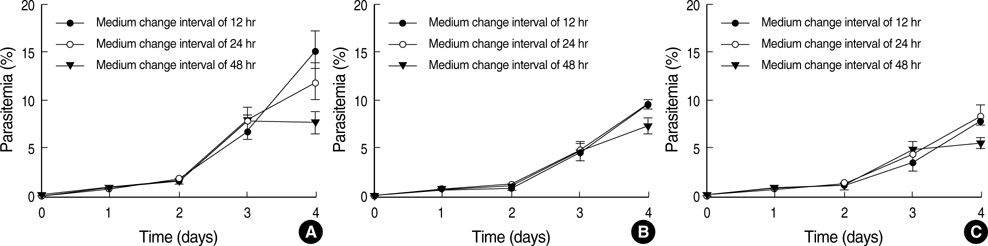
- Differences in the characteristics of the culture conditions can influence the multiplication rate of Plasmodium falciparum. The Petri dish method is one of the most popular methods of cultivating this parasite. In many previous studies, ideal culture conditions of the Petri dish method were achieved by using erythrocytes collected from blood that had been stored for at least 2 weeks, with daily changes of the medium. In the present study, we studied the multiplication rate of P. falciparum in cultures containing erythrocytes of various ages together with changing the medium at various intervals of time. Our results strongly suggest that the rate of in vitro multiplication of P. falciparum was higher in freshly collected erythrocytes than in aged erythrocytes regardless of the anticoagulant and that when the parasitemia is lower than 8% with a hematocrit of 5%, the medium change interval can be as long as 48 hr without a great reduction in the rate of multiplication.
Keyword
MeSH Terms
Figure
Reference
-
1. Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002. 415:680–685.
Article2. Haynes JD, Diggs CL, Hines FA, Desjardins RE. Culture of human malaria parasites, Plasmodium falciparum. Nature. 1976. 263:767–769.
Article3. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976. 193:673–675.
Article4. Jensen JB, Trager W. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle jar method. J Parasitol. 1977. 63:883–886.
Article5. Butcher GA. A comparison of static thin layer and suspension cultures for the maintenance in vitro of Plasmodium falciparum. Ann Trop Med Parasitol. 1981. 75:7–17.6. Trager W. Cultivation of malaria parasites. Methods Cell Biol. 1994. 45:7–26.
Article7. Schuster FL. Cultivation of Plasmodium spp. Clin Microbiol Rev. 2002. 15:355–364.
Article8. Schlichtherle M, Wahlgren M, Perlmann H, Scherf A. Culturing of erythrocytic stages of Plasmodium falciparum parasites. MR4/ATCC, Methods in Malaria Research. 2000. Manassas: Va: 1–7.9. Trager W, Jensen JB. Cultivation of malaria parasites. Nature. 1978. 273:621–622.
Article10. Scheibel LW, Ashton SH, Trager W. Plasmodium falciparum: microaerophilic requirements in human red blood cells. Exp Parasitol. 1979. 47:410–418.
Article11. Jensen JB. Wernsdorfer WH, McGregor I, editors. In vitro cultivation of malaria parasites: erythrocytic stages. Malaria: Principle and Practice of Malariology. 1988. London: Churchill Livingstone;307–320.12. Fairlamb AH, Warhurst DC, Peters W. An improved technique for the cultivation of Plasmodium falciparum in vitro without daily medium change. Ann Trop Med Parasitol. 1985. 79:379–384.
Article13. Ono T, Nakabayashi T. Gametocytogenesis induction by ammonium compounds in cultured Plasmodium falciparum. Int J Parasitol. 1990. 20:615–618.
Article14. Hensmann M, Kwiatkowski D. Cellular basis of early cytokine response to Plasmodium falciparum. Infect Immun. 2001. 69:2364–2371.15. Srinivas SD, Puri SK. Time course of in vitro maturation of intra-erythrocytic malaria parasite: a comparison between Plasmodium falciparum and Plasmodium knowlesi. Mem Inst Oswaldo Cruz. 2002. 97:901–903.
Article16. Wilson RJ, Pasvol G, Weatherall DJ. Invasion and growth of Plasmodium falciparum in different types of human erythrocyte. Bull World Health Organ. 1977. 55:179–186.17. Mons B. Preferential invasion of malarial merozoites into young red blood cells. Blood Cell. 1990. 16:299–312.18. Amaral KF, Rogero MM, Fock RA, Borelli P, Gavini G. Cytotoxicity analysis of EDTA and citric acid applied on murine resident macrophage culture. Int Endod J. 2007. 40:338–343.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gametocytes of Plasmodium falciparum in the megakaryocytes
- A Case of Plasmodium falciparum Gametocytemia Successfully Treated with Primaquine
- Therapeutic RBC Exchange in a Patient with Severe Plasmodium Falciparum Infection
- Treatment outcomes of imported Plasmodium falciparum malaria with intravenous artesunate
- Multiple Cerebral Infarcts Following Acute Plasmodium vivax Infection