Korean J Radiol.
2011 Aug;12(4):456-462. 10.3348/kjr.2011.12.4.456.
The Serum CA-125 Concentration Data Assists in Evaluating CT Imaging Information When Used to Differentiate Borderline Ovarian Tumor from Malignant Epithelial Ovarian Tumors
- Affiliations
-
- 1Department of Radiology and the Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 138-736, Korea. choihj@amc.seoul.kr
- KMID: 1783212
- DOI: http://doi.org/10.3348/kjr.2011.12.4.456
Abstract
OBJECTIVE
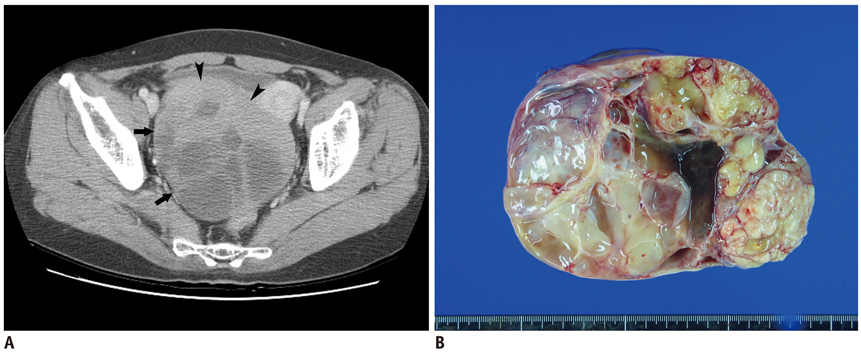
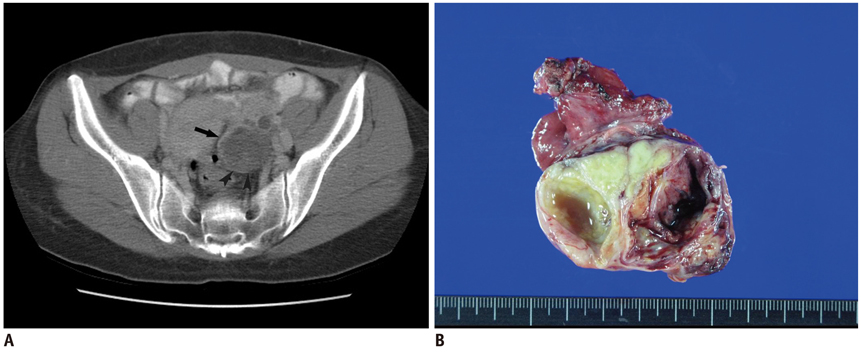
We wanted to evaluate the diagnostic value of serum CA-125 concentration, when used in combination with the preoperative contrast-enhanced CT results, to differentiate borderline ovarian tumors (BOTs) from stage I malignant epithelial ovarian tumors (MEOTs).
MATERIALS AND METHODS
Ninety-eight masses (46 BOTs and 52 stage I MEOTs) from 87 consecutive patients (49 with BOTs and 38 with stage I MEOTs) who had undergone preoperative contrast-enhanced computed tomography (CT) and surgical staging were evaluated retrospectively and independently by two radiologists. The preoperative serum CA-125 concentration was measured in all patients. The utility of analyzing serum CA-125 concentration in combination with the CT results was evaluated by receiver operating characteristic (ROC) curve analysis.
RESULTS
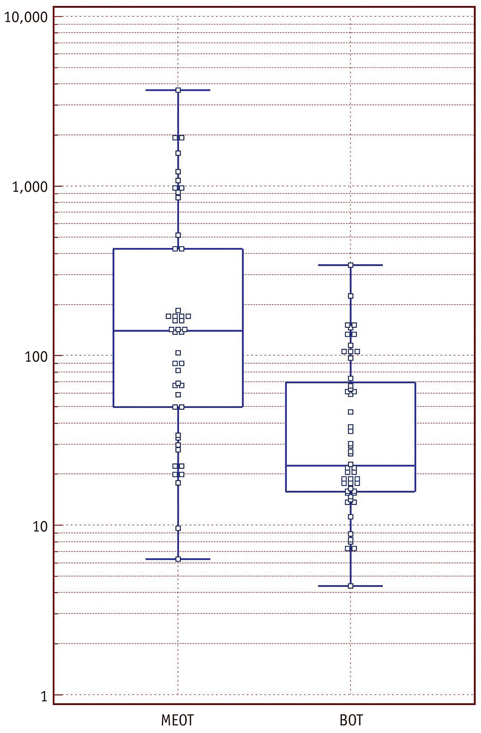
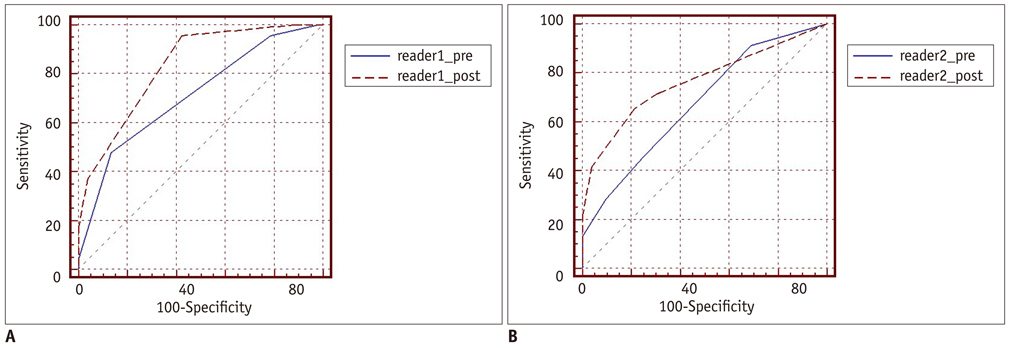
An irregular tumor surface and lymphadenopathy were predictive of a MEOT. ROC analysis showed that the combination of CT data and the serum CA-125 level resulted in a higher diagnostic performance than did using the CT alone for differentiating BOTs from MEOTs. The areas under the curves (AUCs) without and with the use of the serum CA-125 level data were 0.67 (95% confidence interval [CI]: 0.57-0.77) and 0.78 (95% CI: 0.68-0.85), respectively, for reader 1 (p = 0.029) and 0.71 (95% CI: 0.61-0.80) and 0.81 (95% CI: 0.72-0.89), respectively, for reader 2 (p = 0.009).
CONCLUSION
The serum CA-125 concentration is of additional diagnostic value when used in conjunction with the CT imaging results for differentiating BOTs from MEOTs.
Keyword
MeSH Terms
-
Adenocarcinoma, Mucinous/*blood/pathology/*radiography
Adolescent
Adult
Aged
Biological Markers/blood
CA-125 Antigen/*blood
Contrast Media/diagnostic use
Cystadenocarcinoma, Serous/*blood/pathology/*radiography
Diagnosis, Differential
Female
Humans
Middle Aged
Neoplasm Staging
Ovarian Neoplasms/*blood/pathology/*radiography
Predictive Value of Tests
ROC Curve
Retrospective Studies
Figure
Reference
-
1. Jones MB. Borderline ovarian tumors: current concepts for prognostic factors and clinical management. Clin Obstet Gynecol. 2006. 49:517–525.2. Bell DA, Longacre TA, Prat J, Kohn EC, Soslow RA, Ellenson LH, et al. Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Hum Pathol. 2004. 35:934–948.3. Tinelli R, Tinelli A, Tinelli FG, Cicinelli E, Malvasi A. Conservative surgery for borderline ovarian tumors: a review. Gynecol Oncol. 2006. 100:185–191.4. Cadron I, Leunen K, Van Gorp T, Amant F, Neven P, Vergote I. Management of borderline ovarian neoplasms. J Clin Oncol. 2007. 25:2928–2937.5. Holschneider CH, Berek JS. Berek JS, Novak E, editors. Valvar cancer. Berek & Novak's gynecology. 2007. 14th ed. Philadelphia: Lippincott Williams & Wilkins;1549–1580.6. Pignata S, Cannella L, Leopardo D, Pisano C, Bruni GS, Facchini G. Chemotherapy in epithelial ovarian cancer. Cancer Lett. 2011. 303:73–83.7. Buy JN, Ghossain MA, Sciot C, Bazot M, Guinet C, Prevot S, et al. Epithelial tumors of the ovary: CT findings and correlation with US. Radiology. 1991. 178:811–818.8. Dobson M, Carrington BM, Radford JA, Buckley CH, Crowther D. The role of computed tomography in the management of ovarian tumours of borderline malignancy. Clin Radiol. 1997. 52:280–283.9. Grab D, Flock F, Stohr I, Nussle K, Rieber A, Fenchel S, et al. Classification of asymptomatic adnexal masses by ultrasound, magnetic resonance imaging, and positron emission tomography. Gynecol Oncol. 2000. 77:454–459.10. Rieber A, Nussle K, Stohr I, Grab D, Fenchel S, Kreienberg R, et al. Preoperative diagnosis of ovarian tumors with MR imaging: comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Am J Roentgenol. 2001. 177:123–129.11. deSouza NM, O'Neill R, McIndoe GA, Dina R, Soutter WP. Borderline tumors of the ovary: CT and MRI features and tumor markers in differentiation from stage I disease. AJR Am J Roentgenol. 2005. 184:999–1003.12. Van Calster B, Timmerman D, Bourne T, Testa AC, Van Holsbeke C, Domali E, et al. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA-125. J Natl Cancer Inst. 2007. 99:1706–1714.13. Zhang J, Mironov S, Hricak H, Ishill NM, Moskowitz CS, Soslow RA, et al. Characterization of adnexal masses using feature analysis at contrast-enhanced helical computed tomography. J Comput Assist Tomogr. 2008. 32:533–540.14. Brown DL, Zou KH, Tempany CM, Frates MC, Silverman SG, McNeil BJ, et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology. 2001. 219:213–218.15. Choi HJ, Lee JH, Kang S, Seo SS, Choi JI, Lee S, et al. Contrast-enhanced CT for differentiation of ovarian metastasis from gastrointestinal tract cancer: stomach cancer versus colon cancer. AJR Am J Roentgenol. 2006. 187:741–745.16. Jang YJ, Kim JK, Park SB, Cho KS. Variable CT findings of epithelial origin ovarian carcinoma according to the degree of histologic differentiation. Korean J Radiol. 2007. 8:120–126.17. Jung DC, Kim SH. MR imaging findings of ovarian cystadenofibroma and cystadenocarcinofibroma: clues for the differential diagnosis. Korean J Radiol. 2006. 7:199–204.18. Jacobs IJ, Skates S, Davies AP, Woolas RP, Jeyerajah A, Weidemann P, et al. Risk of diagnosis of ovarian cancer after raised serum CA 125 concentration: a prospective cohort study. BMJ. 1996. 313:1355–1358.19. Paramasivam S, Tripcony L, Crandon A, Quinn M, Hammond I, Marsden D, et al. Prognostic importance of preoperative CA-125 in International Federation of Gynecology and Obstetrics stage I epithelial ovarian cancer: an Australian multicenter study. J Clin Oncol. 2005. 23:5938–5942.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extremely elevated serum CA 125 in a borderline tumor of the ovary: A case report
- Diagnostic Significance of Serum Tumor Markers in Paitents with Ovarian Tumors
- A Study on Preoperative Diagnosis in Malignant Ovarian Tumor
- An Immunohistochemical Study of CA 125, CA 19-9, and CA 15-3 in Ovarian Epithelial Tumors
- Significance of Serum CA 125 and CA 15-3 Levels in Patients with Ovarian Tumor