Korean J Radiol.
2011 Aug;12(4):439-449. 10.3348/kjr.2011.12.4.439.
Differentiating Focal Eosinophilic Infiltration from Metastasis in the Liver with Gadoxetic Acid-Enhanced Magnetic Resonance Imaging
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. kshyun@skku.edu
- KMID: 1783210
- DOI: http://doi.org/10.3348/kjr.2011.12.4.439
Abstract
OBJECTIVE
To determine the most useful findings of gadoxetic acid-enhanced 3.0 Tesla (T) MRI for differentiating focal eosinophilic infiltration (FEI) from hepatic metastasis with verification of their usefulness.
MATERIALS AND METHODS
Pathologically or clinically proven 39 FEIs from 25 patients and 79 hepatic metastases from 51 patients were included in the study. Gadoxetic acid-enhanced 3.0T MRI was performed in all cancer patients. Size differences measured between T2-weighted and hepatobiliary-phase images for lesions > 1 cm and morphologic findings (margin, shape, signal intensity on T1- and T2-weighted images, enhancement pattern on dynamic images, and target appearance on hepatobiliary-phase images) were compared between two groups via Student's t test as well as univariate and multivariate analyses. Diagnostic predictive values of two observers for differentiating two groups were assessed before (session 1) and after (session 2) recognition of results.
RESULTS
Mean size difference (2.1 mm) in FEIs between the two images was significantly greater than for metastases (0.7 mm) (p < 0.05). An ill-defined margin and isointensity on T1-weighted images were independently significant morphologic findings (p < 0.05) for differentiating the two groups. All observers achieved a higher diagnostic accuracy in session 2 (97% and 98%) than session 1 (92% and 89%) with statistical significance in observer 2 (p < 0.05). All observers had significantly higher sensitivities (95%) and negative predictive values (NPVs) (98%) in session 2 than in session 1 (sensitivity, 74% in two observers; NPV, 89% and 88%) (p < 0.05).
CONCLUSION
With the size change, an ill-defined margin and isointensity on T1-weighted images are the most useful findings for differentiating FEI from hepatic metastasis on gadoxetic acid-enhanced 3.0T MRI.
MeSH Terms
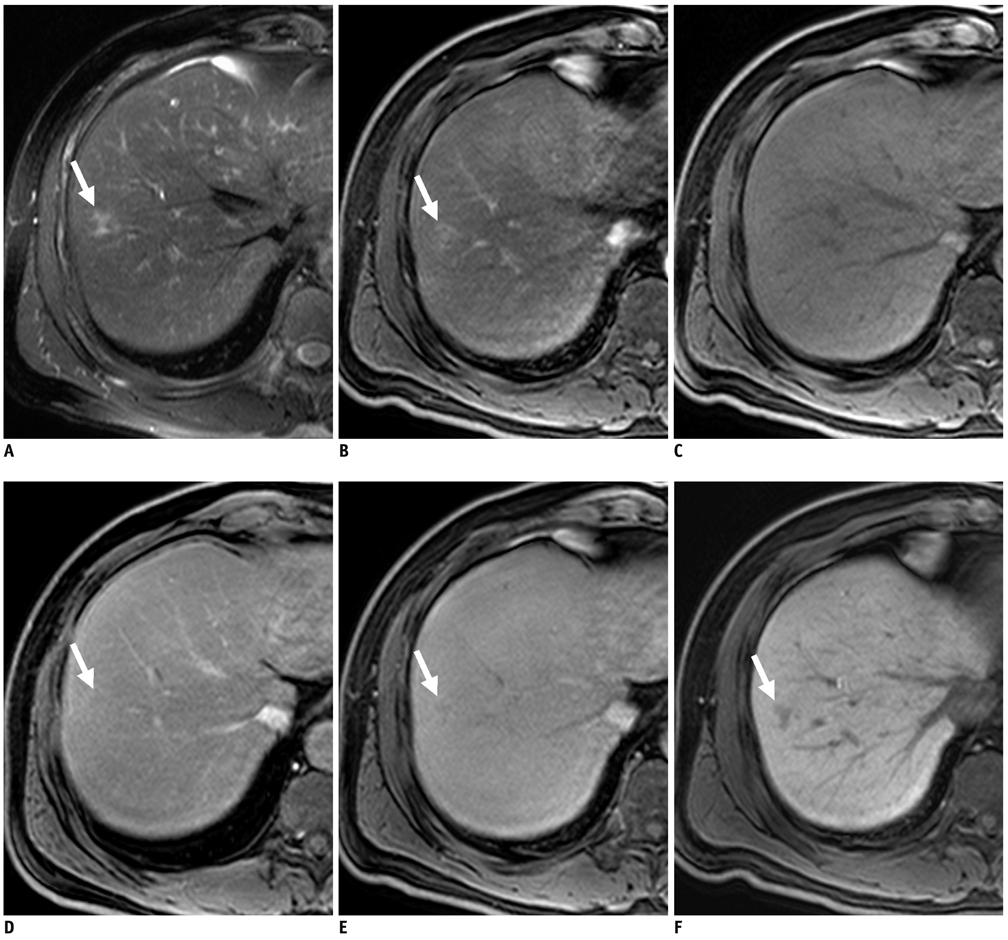
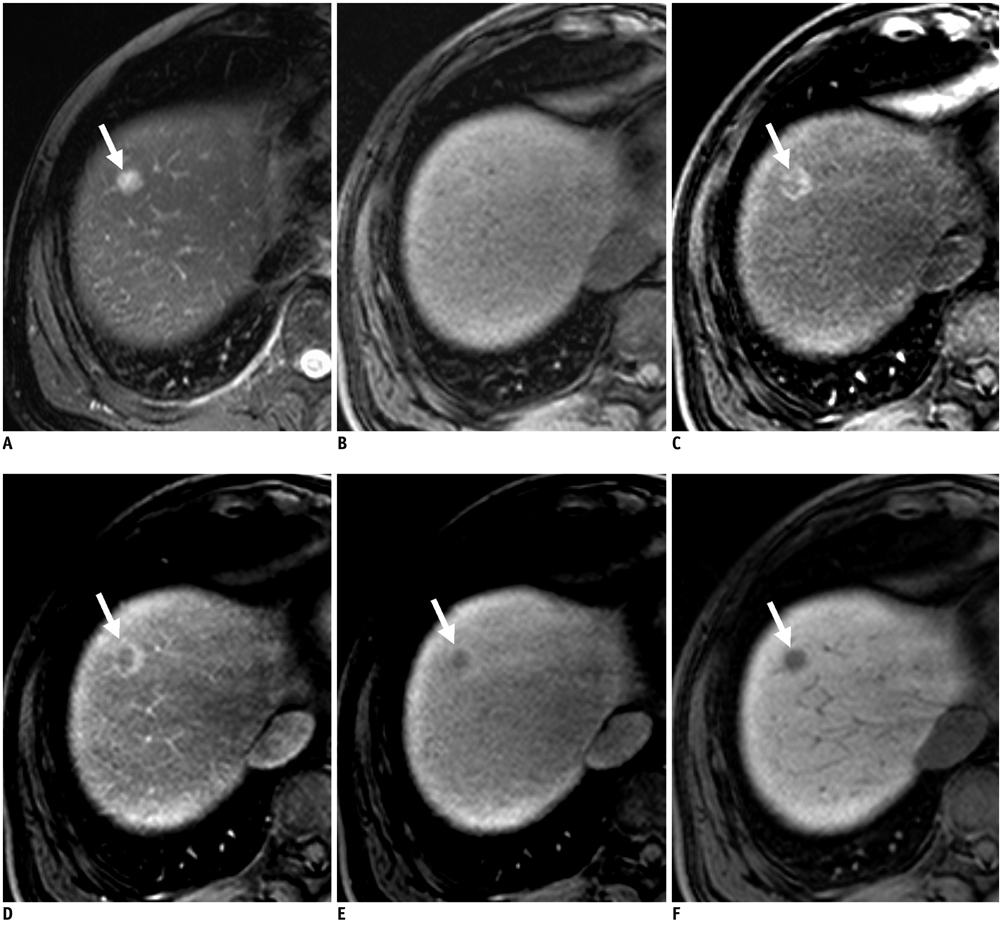
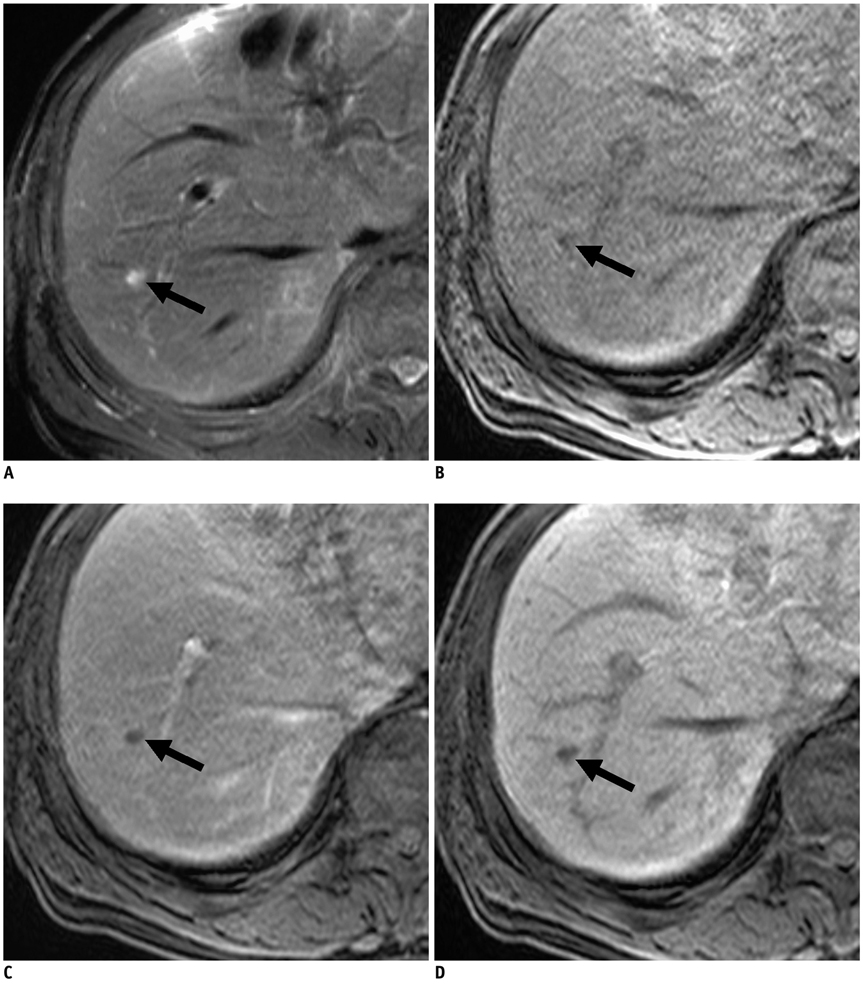
Figure
Cited by 1 articles
-
Hepatic Lesions that Mimic Metastasis on Radiological Imaging during Chemotherapy for Gastrointestinal Malignancy: Recent Updates
Sung-Hye You, Beom Jin Park, Yeul Hong Kim
Korean J Radiol. 2017;18(3):413-426. doi: 10.3348/kjr.2017.18.3.413.
Reference
-
1. Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986. 58:1321–1327.2. Kim GB, Kwon JH, Kang DS. Hypereosinophilic syndrome: imaging findings in patients with hepatic involvement. AJR Am J Roentgenol. 1993. 161:577–580.3. Yoon IL. The eosinophil and gastrointestinal carcinoma. Am J Surg. 1959. 97:195–200.4. Hong SW, Kim HG, Park CI, Lee SI. Eosinophilic liver abscess in patients with gastric carcinoma. Korean J Pathol. 1993. 27:27–33.5. Won JH, Kim MJ, Kim BM, Ji H, Chung JJ, Yoo HS, et al. Focal eosinophilic infiltration of the liver: a mimick of hepatic metastasis. Abdom Imaging. 1999. 24:369–372.6. Hur J, Park MS, Yu JS, Lim JS, Hong SW, Kim KW. Focal eosinophilic necrosis versus metastasis in the liver: the usefulness of two-phase dynamic CT. AJR Am J Roentgenol. 2005. 184:1085–1090.7. Jang HJ, Lee WJ, Lee SJ, Kim SH, Lim HK, Lim JH. Focal eosinophilic necrosis of the liver in patients with underlying gastric or colorectal cancer: CT differentiation from metastasis. Korean J Radiol. 2002. 3:240–244.8. Sun JS, Kim JK, Won JH, Lee KM, Cheong JY, Kim YB. MR findings in eosinophilic infiltration of the liver. J Comput Assist Tomogr. 2005. 29:191–194.9. Yoo SY, Han JK, Kim YH, Kim TK, Choi BI, Han MC. Focal eosinophilic infiltration in the liver: radiologic findings and clinical course. Abdom Imaging. 2003. 28:326–332.10. Yu JS, Yoon SW, Park MS, Lee JH, Kim KW. Eosinophilic hepatic necrosis: magnetic resonance imaging and computed tomography comparison. J Comput Assist Tomogr. 2005. 29:765–771.11. Park MS, Kim MJ, Lim JS, Kim SH, Kim HS, Chung YE, et al. Metastasis versus focal eosinophilic infiltration of the liver in patients with extrahepatic abdominal cancer: an evaluation with gadobenate dimeglumine-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2009. 33:119–124.12. Kim YK, Kim CS, Moon WS, Cho BH, Lee SY, Lee JM. MRI findings of focal eosinophilic liver diseases. AJR Am J Roentgenol. 2005. 184:1541–1548.13. Hamm B, Staks T, Muhler A, Bollow M, Taupitz M, Frenzel T, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995. 195:785–792.14. Bartolozzi C, Crocetti L, Lencioni R, Cioni D, Della Pina C, Campani D. Biliary and reticuloendothelial impairment in hepatocarcinogenesis: the diagnostic role of tissue-specific MR contrast media. Eur Radiol. 2007. 17:2519–2530.15. Spinazzi A, Lorusso V, Pirovano G, Taroni P, Kirchin M, Davies A. Multihance clinical pharmacology: biodistribution and MR enhancement of the liver. Acad Radiol. 1998. 5:Suppl 1. S86–S89. discussion S93-84.16. Jung G, Breuer J, Poll LW, Koch JA, Balzer T, Chang S, et al. Imaging characteristics of hepatocellular carcinoma using the hepatobiliary contrast agent Gd-EOB-DTPA. Acta Radiol. 2006. 47:15–23.17. Halavaara J, Breuer J, Ayuso C, Balzer T, Bellin MF, Blomqvist L, et al. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT--a multicenter trial. J Comput Assist Tomogr. 2006. 30:345–354.18. Bennett BM. On comparisons of sensitivity, specificity and predictive value of a number of diagnostic procedures. Biometrics. 1972. 28:793–800.19. Fleiss JL. The measurement of interrater agreement. Statistical methods for rates and proportions. 1981. New York: John Wiley and Sons;212–236.20. Kanematsu M, Kondo H, Goshima S, Kato H, Tsuge U, Hirose Y, et al. Imaging liver metastases: review and update. Eur J Radiol. 2006. 58:217–228.21. Mahfouz AE, Hamm B, Wolf KJ. Peripheral washout: a sign of malignancy on dynamic gadolinium-enhanced MR images of focal liver lesions. Radiology. 1994. 190:49–52.22. Muramatsu Y, Takayasu K, Moriyama N, Shima Y, Goto H, Ushio K, et al. Peripheral low-density area of hepatic tumors: CT-pathologic correlation. Radiology. 1986. 160:49–52.23. Semelka RC, Hussain SM, Marcos HB, Woosley JT. Perilesional enhancement of hepatic metastases: correlation between MR imaging and histopathologic findings-initial observations. Radiology. 2000. 215:89–94.24. Kim YK, Lee JM, Kim CS. Gadobenate dimeglumine-enhanced liver MR imaging: value of dynamic and delayed imaging for the characterization and detection of focal liver lesions. Eur Radiol. 2004. 14:5–13.25. Gabata T, Matsui O, Kadoya M, Yoshikawa J, Ueda K, Kawamori Y, et al. Delayed MR imaging of the liver: correlation of delayed enhancement of hepatic tumors and pathologic appearance. Abdom Imaging. 1998. 23:309–313.26. Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci. 2007. 6:43–52.27. Danet IM, Semelka RC, Leonardou P, Braga L, Vaidean G, Woosley JT, et al. Spectrum of MRI appearances of untreated metastases of the liver. AJR Am J Roentgenol. 2003. 181:809–817.28. Hwang HS, Kim SH, Jeon TY, Choi D, Lee WJ, Lim HK. Hypointense hepatic lesions depicted on gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR imaging: differentiation between benignancy and malignancy. Korean J Radiol. 2009. 10:294–302.29. Terayama N, Matsui O, Ueda K, Kobayashi S, Sanada J, Gabata T, et al. Peritumoral rim enhancement of liver metastasis: hemodynamics observed on single-level dynamic CT during hepatic arteriography and histopathologic correlation. J Comput Assist Tomogr. 2002. 26:975–980.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gadoxetic acid-enhanced magnetic resonance imaging: Hepatocellular carcinoma and mimickers
- Diagnosis of Hepatocellular Carcinoma with Gadoxetic Acid-Enhanced MRI: 2016 Consensus Recommendations of the Korean Society of Abdominal Radiology
- Hepatic Angiomyolipoma Presenting as a Hyperintense Lesion During the Hepatobiliary Phase of Gadoxetic Acid Enhanced-MRI: a Case Report
- The MR imaging diagnosis of liver diseases using gadoxetic acid: Emphasis on hepatobiliary phase
- Focal Hepatic Eosinophilic Infiltration in Contrast-Enhanced Ultrasonography with Sonazoid: A Case Report