Korean J Radiol.
2005 Jun;6(2):110-116. 10.3348/kjr.2005.6.2.110.
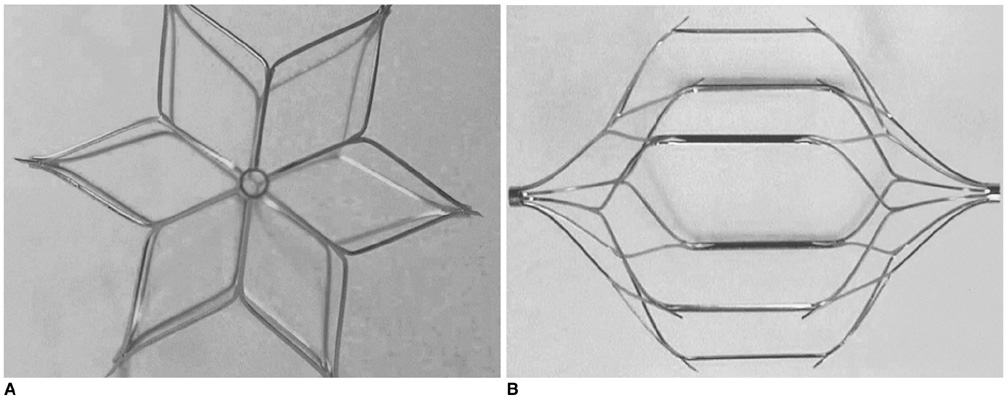
The Mid-Term Efficacy and Safety of a Permanent Nitinol IVC Filter (TrapEase)
- Affiliations
-
- 1Department of Radiology, Sung-Ae General Hospital, korea. ysdo@smc.samsung.co.kr
- 2Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, korea.
- 3Department of Surgery, Cardiovascular Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, korea.
- 4Department of Internal Medicine, Cardiovascular Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, korea.
- KMID: 1783184
- DOI: http://doi.org/10.3348/kjr.2005.6.2.110
Abstract
OBJECTIVE
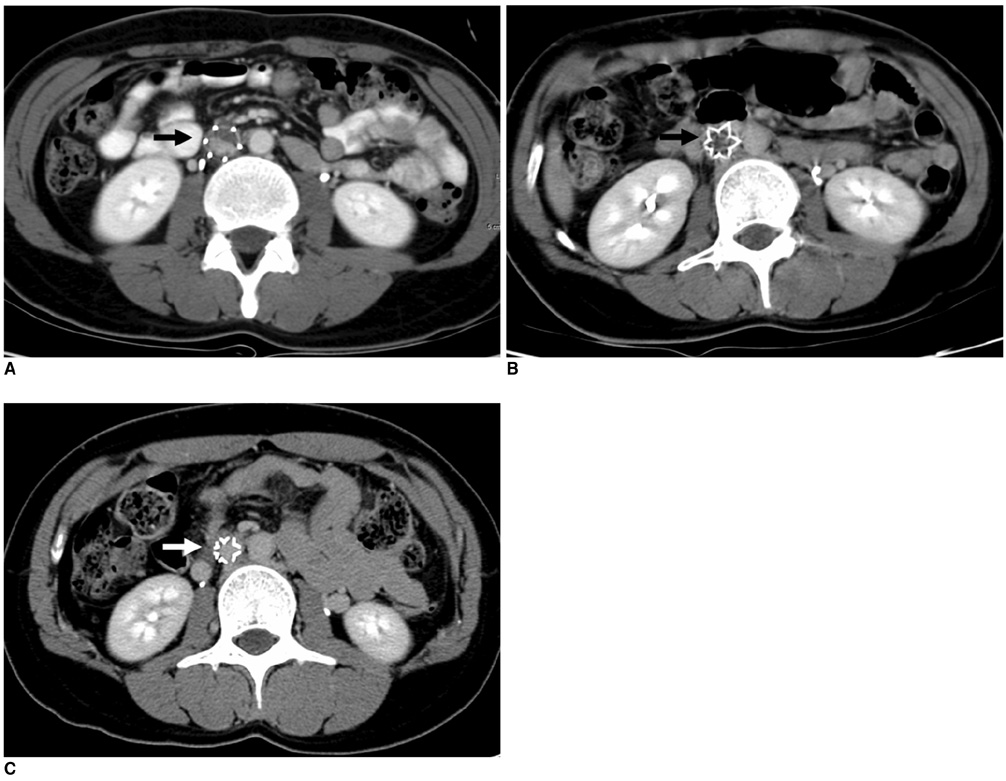
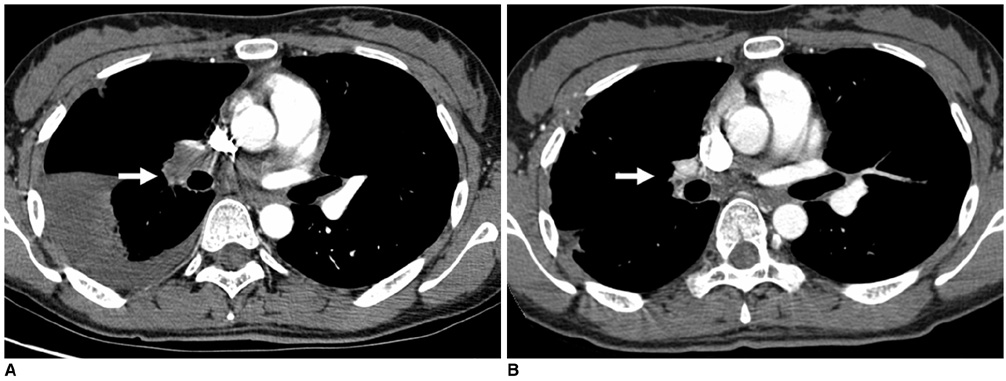
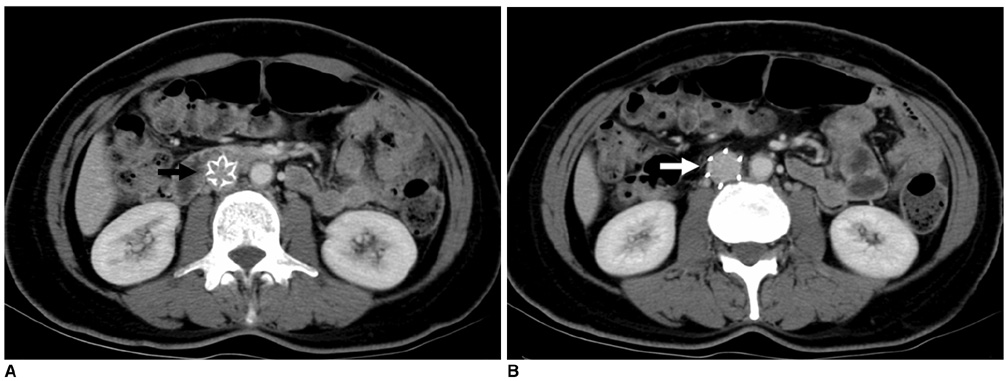
1) To evaluate the mid-term efficacy and safety of a permanent nitinol inferior vena cava (IVC) filter; 2) to evaluate filter effectiveness, filter stability and caval occlusion. MATERIALS AND METHODS: A prospective evaluation of the TrapEase IVC filter was performed on 42 patients (eight men, 34 women) ranging in age from 22 to 78 years (mean age 66 years). All patients were ill with a high risk of pulmonary embolism (PE). Indications for filter placement were: 1) deep vein thrombosis with recurrent thromboembolism; 2) and/or free-floating thrombus with contraindication to anticoagulation; and 3) complications in achieving adequate anticoagulation. Follow-up evaluations (mean: 15.4 months, range: 2 to 28 months) were performed at 6- and 12-month intervals after the procedure and included clinical histories, chart reviews, plain film, Doppler ultrasounds, and contrasted abdominal CT scans. RESULTS: In follow-up evaluations, the data analysis revealed no cases of symptomatic PE. There were no cases of filter migration, insertion site thrombosis, filter fracture, or vessel wall perforation. During the study, there was one case of filter thrombosis; early symptomatic thrombosis that was successfully treated in the hospital. Of the 42 subjects, eight died. These deaths were not related to the filter device or the implantation procedure, but to the underlying disease. CONCLUSION: This study demonstrates that the TrapEase permanent IVC filter is a safe and an effective device with low complication rates and is best used in patients with thromboembolic disease with a high risk of PE.
MeSH Terms
Figure
Reference
-
1. Ferris EJ, McCowan TC, Carver DK, McFarland DR. Percutaneous inferior vena caval filters: follow-up of seven designs in 320 patients. Radiology. 1993. 188:851–856.2. Rousseau H, Perreault P, Otal P, Stockx L, Golzarian J, Oliva V, et al. The 6-F nitinol TrapEase inferior vena cava filter: results of a prospective multicenter trial. J Vasc Interv Radiol. 2001. 12:299–304.3. Grassi CJ. Inferior vena caval filters: analysis of five currently available devices. AJR Am J Roentgenol. 1991. 156:813–821.4. Tardy B, Mismetti P, Page Y, Decouses H, Da Costa A, Zeni F, et al. Symptomatic inferior vena cava filter thrombosis: clinical study of 30 consecutive cases. Eur Respir J. 1996. 9:2012–2016.5. Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000. 95:3669–3677.6. Mewissen MW, Erickson SJ, Foley WD, Lipchik EO, Olson DL, McCann KM, et al. Thrombosis at venous insertion sites after inferior vena caval filter placement. Radiology. 1989. 173:155–157.7. Kantor A, Glanz S, Gordon DH, Sclafani SJ. Percutaneous insertion of the Kimray-Greenfield filter: incidence of femoral vein thrombosis. AJR Am J Roentgenol. 1987. 149:1065–1066.8. Simon M, Athanasoulis CA, Kim D, Steinberg FL, Porter DH, Byse BH, et al. Simon nitinol inferior vena cava filter: initial clinical experience. Work in progress. Radiology. 1989. 172:99–103.9. Schleich JM, Morla O, Laurent M, Langella B, Chaperon J, Almange C. Long-term follow-up of percutaneous vena cava filters: a prospective study in 100 consecutive patients. Eur J Vasc Endovasc Surg. 2001. 21:450–457.10. Kinney TB. Update on inferior vena cava filters. J Vasc Interv Radiol. 2003. 14:425–440.11. Rose BS, Simon DC, Hess ML, Van Aman ME. Percutaneous transfemoral placement of the Kimray-Greenfield vena cava filter. Radiology. 1987. 165:373–376.12. Ricco JB, Crochet D, Sebilotte P, Serradimigni A, Lefebvre JM, Bouissou E, et al. Percutaneous transvenous caval interruption with the "LGM" filter: early results of a multicenter trial. Ann Vasc Surg. 1988. 2:242–247.13. Greenfield LJ, Cho KJ, Tauscher JR. Limitations of percutaneous insertion of Greenfield filters. J Cardiovasc Surg (Torino). 1990. 31:344–350.14. Schneider PA, Geissbuhler P, Piguet JC, Bounameaux H. Follow-up after partial interruption of the vena cava with the Gunther filter. Cardiovasc Intervent Radiol. 1990. 13:378–380.15. Athanasoulis CA, Kaufman JA, Halpern EF, Waltman AC, Geller SC, Fan CM. Inferior vena caval filters: review of a 26-year single-center clinical experience. Radiology. 2000. 216:54–66.16. Sweeney TJ, Van Aman ME. Deployment problems with the titanium Greenfield filter. J Vasc Interv Radiol. 1993. 4:691–694.17. Roehm JO Jr, Johnsrude IS, Barth MH, Gianturco C. The bird's nest inferior vena cava filter: progress report. Radiology. 1988. 168:745–749.18. Cimochowski GE, Evans RH, Zarins CK, Lu CT, DeMeester TR. Greenfield filter versus Mobin-Uddin umbrella: the continuing quest for the ideal method of vena caval interruption. J Thorac Cardiovasc Surg. 1980. 79:358–365.19. Greenfield LJ, Cho KJ, Proctor M, Bonn J, Bookstein JJ, Castaneda-Zuniga WR, et al. Results of a multicenter study of the modified hook-titanium Greenfield filter. J Vasc Surg. 1991. 14:253–257.20. Brenner DW, Brenner CJ, Scott J, Wehberg K, Granger JP, Schellhammer PF. Suprarenal Greenfield filter placement to prevent pulmonary embolus in patients with vena caval tumor thrombi. J Urol. 1992. 147:19–23.21. Proctor MC, Greenfield LJ, Cho KJ, Moursi MM, James EA. Assessment of apparent vena caval penetration by the Greenfield filter. J Endovasc Surg. 1998. 5:251–258.22. Nicolaides AN, Arcelus J, Belcaro G, Bergqvist D, Borris LC, Buller HR, et al. Prevention of venous thromboembolism. European Consensus Statement, 1-5 November 1991, developed at Oakley Court Hotel, Windsor, UK. Int Angiol. 1992. 11:151–159.23. Paiement GD, Desautels C. Deep vein thrombosis: prophylaxis, diagnosis, and treatment--lessons from orthopedic studies. Clin Cardiol. 1990. 13(4):Suppl 6. VI19–VI22.24. Stavropoulos SW, Clark T, Jacobs D, Soulen M, Shlansky-Goldberg R, Solomon J, et al. Placement of a vena cava filter with an antecubital approach. Acad Radiol. 2002. 9:478–481.25. Lord RS, Benn I. Early and late results after Bird's Nest filter placement in the inferior vena cava: clinical and duplex ultrasound follow-up. Aust N Z J Surg. 1994. 64:106–114.26. Poletti PA, Becker CD, Prina L, Ruijs P, Bounameaux H, Didier D, et al. Long-term results of the Simon nitinol inferior vena cava filter. Eur Radiol. 1998. 8:289–294.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Contributing to Inferior Vena Cava Filter Removal Failure using Advanced Techniques: A Single-Center Study
- Outcomes of Inferior Vena Cava Filter Insertion in Patients with Lower Extremity Deep Vein Thrombosis for Prevention of Pulmonary Thromboembolism: A Single Center Retrospective Analysis
- Fracture and Embolization of a Celect Inferior Vena Cava Filter Strut to the Liver: A Case Report
- Spontaneous Tilting after Placement of the Gunther-Tulip Inferior Vena Caval Filter: A Case Report
- Surgical Removal of the Inferior Vena Cava Filter Using Minimal Cavotomy: A Case Report