J Korean Med Sci.
2009 Dec;24(6):1203-1206. 10.3346/jkms.2009.24.6.1203.
Fibrinogen Yecheon: Congenital Dysfibrinogenemia with Gamma Methionine-310 to Threonine Substitution
- Affiliations
-
- 1Department of Internal Medicine, Hemato-oncology, Chung-Ang University Hospital, Seoul, Korea.
- 2Department of Laboratory Medicine, Chung-Ang University Hospital, Seoul, Korea. chayoung@cau.ac.kr
- 3Department of Laboratory Medicine, Soonchunhyang University Hospital, Seoul, Korea.
- 4Department of Laboratory Medicine, Samsung Medical Center, Seoul, Korea.
- KMID: 1783157
- DOI: http://doi.org/10.3346/jkms.2009.24.6.1203
Abstract
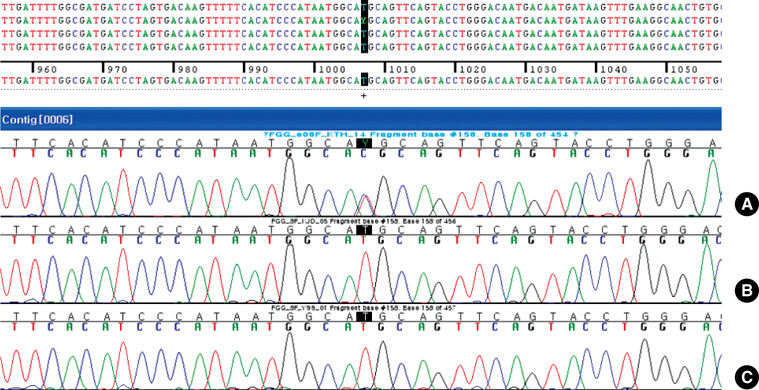
- This case study reports a rare fibrinogen variant, gamma Met310Thr mutation, for the first time in Korea. The case shows a point mutation from T to C in the 1,007th nucleotide of the FGG gene. This report describes a variant fibrinogen, hereinafter called "fibrinogen Yecheon", using the name after the town where the patient was living at the time of diagnosis. Fibrinogen Yecheon has a de novo heterozygous point mutation of FGG resulting in gamma Met310Thr and subsequent extra N-glycosylation at gamma Asn308. Extra N-glycosylated fibrinogen is considered a main inhibitor of normal fibrinogen activity.
MeSH Terms
Figure
Reference
-
1. Cote HC, Lord ST, Pratt KP. Gamma-chain dysfibrinogenemias: molecular structure-function relationships of naturally occurring mutations in the gamma chain of human fibrinogen. Blood. 1998. 92:2195–2212.2. Hanss M, Biot F. A database for human fibrinogen variants. Ann N Y Acad Sci. 2001. 936:89–90.
Article3. Kang HG, Bybee A, Ha IS, Park MS, Gilbertson JA, Cheong HI, Choi Y, Hawkins PN. Hereditary amyloidosis in early childhood associated with a novel insertion-deletion (indel) in the fibrinogen Aalpha chain gene. Kidney Int. 2005. 68:1994–1998.4. Song KS, Park NJ, Choi JR, Doh HJ, Chung KH. Fibrinogen Seoul (FGG Ala341Asp): a novel mutation associated with hypodysfibrinogenemia. Clin Appl Thromb Hemost. 2006. 12:338–343.
Article5. Park R, Doh HJ, An SS, Choi JR, Chung KH, Song KS. A novel fibrinogen variant (fibrinogen Seoul II; AalphaGln328Pro) characterized by impaired fibrin alpha-chain cross-linking. Blood. 2006. 108:1919–1924.6. Yamazumi K, Shimura K, Terukina S, Takahashi N, Matsuda M. A gamma methionine-310 to threonine substitution and consequent N-glycosylation at gamma asparagine-308 identified in a congenital dysfibrinogenemia associated with posttraumatic bleeding, fibrinogen Asahi. J Clin Invest. 1989. 83:1590–1597.
Article7. Galanakis D, Spitzer S, Scharrer I, Peerschke E. Impaired platelet aggregation support by two dysfibrinogene: a gamma319-320 deletion and a gamma310Met->Thr substitution. Thromb Haemost. 1993. 69:1261.8. Yamazumi K, Shimura K, Maekawa H, Muramatsu S, Terukina S, Matsuda M. Delayed intermolecular gamma-chain cross-linking by factor XIIIa in fibrinogen Asahi characterized by a gamma-Met-310 to Thr substitution with an N-glycosylated gamma-Asn-308. Blood Coagul Fibrinolysis. 1990. 1:557–559.9. Woodhead JL, Nagaswami C, Matsuda M, Arocha-Pinango CL, Weisel JW. The ultrastructure of fibrinogen Caracas II molecules, fibers, and clots. J Biol Chem. 1996. 271:4946–4953.
Article10. Townsend RR, Hilliker E, Li YT, Laine RA, Bell WR, Lee YC. Carbohydrate structure of human fibrinogen. Use of 300-MHz 1H-NMR to characterize glycosidase-treated glycopeptides. J Biol Chem. 1982. 257:9704–9710.
Article11. Nishibe H, Takahashi N. The release of carbohydrate moieties from human fibrinogen by almond glycopeptidase without alteration in fibrinogen clottability. Biochimica et biophysica acta. 1981. 661:274–279.
Article12. Sugo T, Nakamikawa C, Takano H, Mimuro J, Yamaguchi S, Mosesson M, Meh D, DiOrio JP, Takahashi N, Takahashi H, Nagai K, Matsuda M. Fibrinogen Niigata with impaired fibrin assembly: an inherited dysfibrinogen with a Bbeta Asn-160 to Ser substitution associated with extra glycosylation at Bbeta Asn-158. Blood. 1999. 94:3806–3813.13. Sugo T, Sekine O, Nakamikawa C, Endo H, Arocha-Pinango CL, Matsuda M. Mode of perturbation of Asahi fibrin assembly by the extra oligosaccharides. Ann N Y Acad Sci. 2001. 936:223–225.
Article14. Maekawa H, Yamazumi K, Muramatsu S, Kaneko M, Hirata H, Takahashi N, Arocha-Pinango CL, Rodriguez S, Nagy H, Perez-Requejo JL. Fibrinogen Lima: a homozygous dysfibrinogen with an A alpha-arginine-141 to serine substitution associated with extra N-glycosylation at A alpha-asparagine-139. Impaired fibrin gel formation but normal fibrin-facilitated plasminogen activation catalyzed by tissue-type plasminogen activator. J Clin Invest. 1992. 90:67–76.
Article15. Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M, Susser E. Advancing paternal age and autism. Arch Gen Psychiatry. 2006. 63:1026–1032.
Article16. Yauk C, Polyzos A, Rowan-Carroll A, Somers CM, Godschalk RW, Van Schooten FJ, Berndt ML, Pogribny IP, Koturbash I, Williams A, Douglas GR, Kovalchuk O. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc Natl Acad Sci USA. 2008. 105:605–610.
Article17. Hu C, Jiang L, Geng C, Zhang X, Cao J, Zhong L. Possible involvement of oxidative stress in trichloroethylene-induced genotoxicity in human HepG2 cells. Mutat Res. 2008. 652:88–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biochemical Characteristics of Dysfunctional Fibrinogen Found in Korea
- Spleen Rupture in Congenital Afibrinogenemia
- Lack of Association between Angiotensinogen M235T Gene Polymorphism and Ischemic Stroke in Korean
- Mutations at the Gene Encoding the 'a' Determinant of HBsAg in Chronic Hepatitis B Patients with Concurrent HBsAg and Anti-HBs Positivity
- Studies on preparation of 99mTc complexes of methionine isomers