J Korean Med Sci.
2009 Dec;24(6):1024-1030. 10.3346/jkms.2009.24.6.1024.
Toxocariasis Might be an Important Cause of Atopic Myelitis in Korea
- Affiliations
-
- 1Department of Medicine, Center for Health Promotion, Samsung Medical Center, Seoul, Korea.
- 2Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Medicine, Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea.
- 4Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dongchull.choi@samsung.com
- 5Department of Medicine, Bundang Jesaeng Hospital, Seongnam, Korea.
- 6Samsung Biomedical Research Institute, Seoul, Korea.
- KMID: 1783128
- DOI: http://doi.org/10.3346/jkms.2009.24.6.1024
Abstract
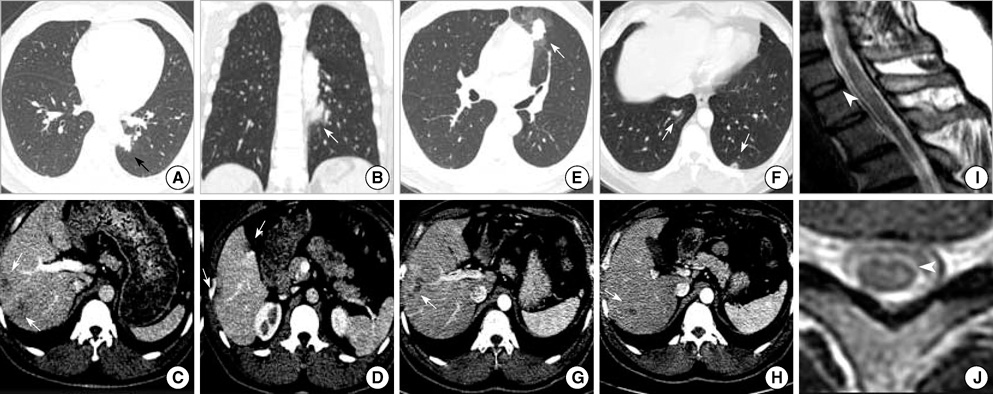
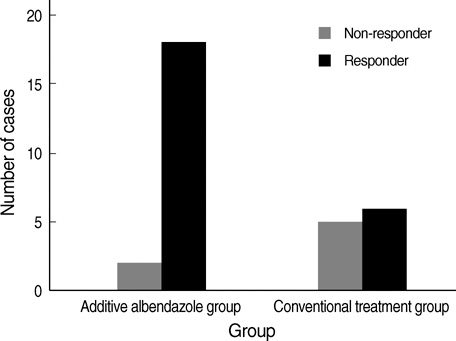
- Atopic myelitis is defined as myelitis with atopic diasthesis but the cause is still unknown. Toxocariasis is one of the common causes of hyperIgEaemia that may lead to neurologic manifestations. The purpose of this study was to evaluate the sero-prevalence of Toxocara specific IgG Ab among the atopic myelitis patients. We evaluated the medical records of 37 patients with atopic myelitis whose conditions were diagnosed between March 2001 and August 2007. Among them, the 33 sera were analyzed for specific serum IgG Ab to Toxocara excretory-secretory antigens (TES). All of 37 patients had hyperIgEaemia. Specific IgE to D. pteronyssinus and D. farinae was detected in 22 (64.7%) and 34 (100%) patients, respectively, of the 34 patients. Thirty-one of 33 patients (93.9%) were found to be positive by TES IgG enzyme-linked immunosorbent assay (ELISA). Based on the image findings of eosinophilic infiltrations in the lung and liver, 8 patients had positive results. These results inferred that the prevalence of toxocariasis was high in patients with atopic myelitis. Our results suggest that toxocariasis might be an important cause of atopic myelitis and Toxocara ELISA is essential for evaluating the causes of atopic myelitis.
Keyword
MeSH Terms
-
Adult
Albendazole/therapeutic use
Animals
Anthelmintics/therapeutic use
Antibodies, Helminth/blood/immunology
Humans
Immunoglobulin E/blood/immunology
Magnetic Resonance Imaging
Middle Aged
Myelitis/drug therapy/*etiology/*immunology/pathology
Retrospective Studies
Toxocara/*immunology
Toxocariasis/*complications/drug therapy/*immunology/pathology
Treatment Outcome
Young Adult
Figure
Cited by 1 articles
-
Eosinophilic Myelitis in the Cervical Cord Mimicking Intramedullary Cord Tumor
Cheon Wook Park, Woo Jin Choe, Young Il Chun
J Korean Neurosurg Soc. 2012;52(4):410-413. doi: 10.3340/jkns.2012.52.4.410.
Reference
-
1. Osoegawa M, Ochi H, Minohara M, Murai H, Umehara F, Furuya H, Yamada T, Kira J. Myelitis with atopic diathesis: a nationwide survey of 79 cases in Japan. J Neurol Sci. 2003. 209:5–11.
Article2. Kira J, Yamasaki K, Kawano Y, Kobayashi T. Acute myelitis associated with hyperIgEemia and atopic dermatitis. J Neurol Sci. 1997. 148:199–203.
Article3. Kira J, Horiuchi I, Suzuki J, Osoegawa M, Tobimatsu S, Murai H, Minohara M, Furue M, Ochi H. Myelitis associated with atopic disorders in Japan: a retrospective clinical study of the past 20 years. Intern Med. 2001. 40:613–619.
Article4. Osoegawa M, Ochi H, Kikuchi H, Shirabe S, Nagashima T, Tsumoto T, Tamura Y, Yamabe K, Takahashi H, Iwaki T, Kira J. Eosinophilic myelitis associated with atopic diathesis: a combined neuroimaging and histopathological study. Acta Neuropathol. 2003. 105:289–295.
Article5. Kira J. Atopy and neural damage. Intern Med. 2002. 41:169–174.
Article6. Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006. 85:233–238.
Article7. Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001. 39:1–11.
Article8. Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981. 3:230–250.
Article9. de Savigny DH, Voller A, Woodruff AW. Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol. 1979. 32:284–288.
Article10. Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991. 29:1831–1835.
Article11. Wang C, Huang CY, Chan PH, Preston P, Chau PY. Transverse myelitis associated with larva migrans: finding of larva in cerebrospinal fluid. Lancet. 1983. 1:423.
Article12. Park HY, Lee SU, Huh S, Kong Y, Magnaval JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol. 2002. 40:113–117.
Article13. Magnaval JF, Galindo V, Glickman LT, Clanet M. Human Toxocara infection of the central nervous system and neurological disorders: a case-control study. Parasitology. 1997. 115:537–543.
Article14. Strupp M, Pfister HW, Eichenlaub S, Arbusow V. Meningomyelitis in a case of toxocariasis with markedly isolated CSF eosinophilia and an MRI-documented thoracic cord lesion. J Neurol. 1999. 246:741–744.
Article15. Eberhardt O, Bialek R, Nagele T, Dichgans J. Eosinophilic meningomyelitis in toxocariasis: case report and review of the literature. Clin Neurol Neurosurg. 2005. 107:432–438.
Article16. Kumar J, Kimm J. MR in Toxocara canis myelopathy. AJNR Am J Neuroradiol. 1994. 15:1918–1920.17. Russegger L, Schmutzhard E. Spinal toxocaral abscess. Lancet. 1989. 2:398.
Article18. Engel H, Spieckermann DA, Tismer R, Mobius W. Acute meningomyelitis due to Toxocara larvae. Dtsch Med Wochenschr. 1971. 96:1498.19. Muller-Jensen A, Schall J, Weisner B, Lamina J. Eosinophilic meningoencephalomyelitis and visceral syndrome caused by ascaridia larvae in adults. Dtsch Med Wochenschr. 1973. 98:1175–1177.20. Sellal F, Picard F, Mutschler V, Marescaux C, Collard M, Magnaval JF. Myelitis caused by Toxocara canis (larva migrans). Rev Neurol (Paris). 1992. 148:53–55.21. Ota S, Komiyama A, Johkura K, Hasegawa O, Kondo K. Eosinophilic meningo-encephalo-myelitis due to Toxocara canis. Rinsho Shinkeigaku. 1994. 34:1148–1152.22. Duprez TP, Bigaignon G, Delgrange E, Desfontaines P, Hermans M, Vervoort T, Sindic CJ, Buysschaert M. MRI of cervical cord lesions and their resolution in Toxocara canis myelopathy. Neuroradiology. 1996. 38:792–795.
Article23. Goffette S, Jeanjean AP, Duprez TP, Bigaignon G, Sindic CJ. Eosinophilic pleocytosis and myelitis related to Toxocara canis infection. Eur J Neurol. 2000. 7:703–706.24. Osoegawa M. Diagnosis and treatment of CNS parasite infection with special reference to parasitic myelitis. Rinsho Shinkeigaku. 2004. 44:961–964.25. Dauriac-Le Masson V, Chochon F, Demeret S, Pierrot-Deseilligny C. Toxocara canis meningomyelitis. J Neurol. 2005. 252:1267–1268.
Article26. Moreira-Silva SF, Rodrigues MG, Pimenta JL, Gomes CP, Freire LH, Pereira FE. Toxocariasis of the central nervous system: with report of two cases. Rev Soc Bras Med Trop. 2004. 37:169–174.
Article27. Chang S, Lim JH, Choi D, Park CK, Kwon NH, Cho SY, Choi DC. Hepatic visceral larva migrans of Toxocara canis: CT and sonographic findings. AJR Am J Roentgenol. 2006. 187:W622–W629.28. Magnaval JF, Fabre R, Maurieres P, Charlet JP, de Larrard B. Application of the western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol Res. 1991. 77:697–702.
Article29. Kennedy MW, Tierney J, Ye P, McMonagle FA, McIntosh A, Mc-Laughlin D, Smith JW. The secreted and somatic antigens of the third stage larva of Anisakis simplex, and antigenic relationship with Ascaris suum, Ascaris lumbricoides, and Toxocara canis. Mol Biochem Parasitol. 1988. 31:35–46.
Article30. Johansson E, Aponno M, Lundberg M, van Hage-Hamsten M. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy. 2001. 56:660–666.
Article