Korean J Hematol.
2011 Jun;46(2):118-122. 10.5045/kjh.2011.46.2.118.
Hemoglobin F level in different hemoglobin variants
- Affiliations
-
- 1Division of Hematology, Department of Biomedical Science, Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria. olufemiakanni@yahoo.com
- 2Department of Hematology and Blood Transfusion, Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria.
- 3Department of Physiology, College of Medicine, University of Ibadan, Ibadan, Nigeria.
- KMID: 1782776
- DOI: http://doi.org/10.5045/kjh.2011.46.2.118
Abstract
- BACKGROUND
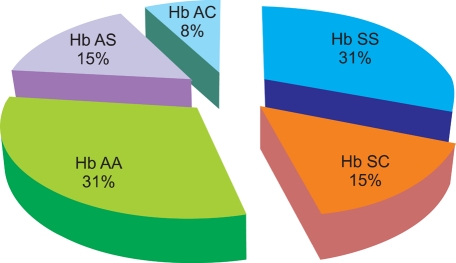
Fetal hemoglobin (HbF) levels in different hemoglobin variants in Osogbo, Nigeria, were estimated using two principal methods of estimation using existing information for HbF concentration and distribution of various hemoglobin variants in the area, as well as diagnosed cases of thalassemia. Two hundred and sixty samples collected from HbSS, HbSC, HbAA, HbAS, and HbAC subjects were analyzed. HbF level and hemoglobin type were determined in this study.
METHODS
The hemoglobin type was determined using cellulose acetate electrophoresis at an alka-line pH, and HbF was determined by the acid elution and alkaline denaturation methods.
RESULTS
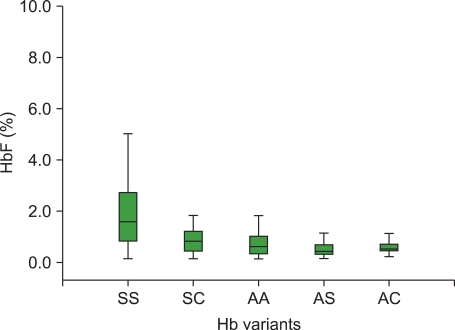
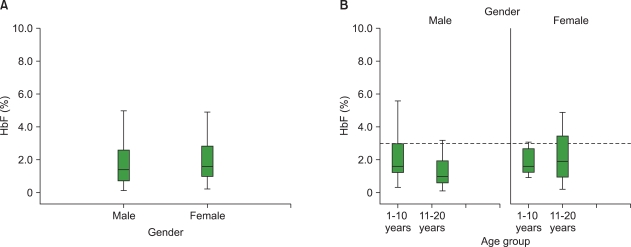
The mean+/-SD of HbF in the respective hemoglobin variants was as follows: HbSS, 2.09+/-1.94%; HbSC, 0.85+/-0.54%; HbAA, 0.69+/-0.46%; HbAS, 0.52+/-0.31%; and HbAC, 0.57+/-0.26%. The mean HbF level across the hemoglobin variants was statistically significant (P<0.05). Investigating the sex distribution of the HbF level in the studied population revealed a slightly higher mean HbF level in females than in their male counterparts.
CONCLUSION
Within the study population, the HbF level was found to be highest in HbSS and lowest in HbAS. The two methods of estimating HbF are equally reliable, since there was no significant difference between the results obtained from the two methods.
Keyword
MeSH Terms
Figure
Reference
-
1. Weatherall DJ. Hoffbrand AV, Lewis SM, Tuddenham EG, editors. Genetic disorders of haemoglobin. Postgrauate haematology. 1999. 4th ed. Oxford, UK: Butterworth-Heinemann;p. 91–119.2. Wood WG. Increased HbF in adult life. Baillieres Clin Haematol. 1993; 6:177–213. PMID: 7688998.3. Kan YW, Holland JP, Dozy AM, Charache S, Kazazian HH. Deletion of the beta-globin structure gene in hereditary persistence of foetal haemoglobin. Nature. 1975; 258:162–163. PMID: 1186896.4. Kotila TR, Fawole OI, Shokunbi WA. Haemoglobin F and clinical severity of sickle cell anaemia among Nigerian adults. Afr J Med Med Sci. 2000; 29:229–231. PMID: 11713996.5. Harmening DM, Lasky L, Latchaw P. Harmening DM, editor. Blood preservation: historical perspectives, review of metabolism and current trends. Modern blood banking and transfusion practices. 1999. 4th ed. Philadelphia, PA: F.A. Davis co..6. Cheesbrough M, editor. District laboratory practice in Tropical Countries, Part 1. 2006. 2nd ed. Cambridge, UK: Cambridge University press;p. 268–285.7. Kleihauer E, Braun H, Betke K. Dacie JV, Lewis SM, editors. Erythrocyte and leukocyte cytochemistry-leukaemia classification. Practical haematology. 2001. 9th ed. London, UK: Curchill Livingstone;p. 269–295.8. Hoffbrand AV, Pettit JE, Moss PAH. Essential haematology. 2001. 4th ed. Oxford, UK: Blackwell Science;p. 71–90.9. Bunn HF. Stamatoyannopoulos G, Nienhuis AW, Majerus PW, Varmus H, editors. Sickle hemoglobin and other hemoglobin mutants. The molecular basis of blood diseases. 1994. 2nd ed. Philadelphia, PA: W.B. Saunders;p. 207–256.10. Corda M, De Rosa MC, Pellegrini MG, et al. Adult and fetal haemoglobin J-Sardegna [alpha50(CE8)His→Asp]: functional and molecular modelling studies. Biochem J. 2000; 346:193–199. PMID: 10657257.11. Patrinos GP, Giardine B, Riemer C, et al. Improvements in the HbVar database of human hemoglobin variants and thalassemia mutations for population and sequence variation studies. Nucleic Acids Res. 2004; 32:D537–D541. PMID: 14681476.
Article12. De Rosa MC, Alinovi CC, Schinina ME, et al. Hb Santa Clara (beta 97His→Asn), a human haemoglobin variant: functional characterization and structure modelling. Biochim Biophys Acta. 2007; 1774:1299–1306. PMID: 17881306.13. Cheesbrough M, editor. ICSH: International Committee for Standardization in Haematology. Haematological Tests. District laboratory practice in Tropical Countries, Part 1. 1988. Cambridge, UK: Cambridge University press;p. 282–298.14. Betke K, Mati HR, Schlicht L. Dacie JV, Lewis SM, editors. Investigation of abnormal haemoglobins and thalassaemia. Practical haematology. 2001. 9th ed. London, UK: Curchill Livingstone;p. 231–268.15. Falusi AG, Esan GJ. Foetal haemoglobin levels in sickle cell anaemia in Nigerians. Afr J Med Med Sci. 1989; 18:145–149. PMID: 2474239.16. Uko EK, Useh MF, Gwanmesia FN. Frequency of foetal haemoglobin and haemoglobin values in various haemoglobin genotypes in Calabar, Nigeria. East Afr Med J. 1997; 74:809–811. PMID: 9557428.17. Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A. 2008; 105:1620–1625. PMID: 18245381.18. Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008; 322:1839–1842. PMID: 19056937.
Article19. Mason KP, Grandison Y, Hayes RJ, et al. Post-natal decline of fetal haemoglobin in homozygous sickle cell disease: relationship to parenteral Hb F levels. Br J Haematol. 1982; 52:455–463. PMID: 6181802.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Hemoglobin Level on the Diffusing Capacity of the Lungs
- Distribution of Hemoglobin Concentration before Transfusion for Evaluation of Appropriateness of Red Blood Cell Transfusion
- The Combination of Fasting Plasma Glucose and Glycosylated Hemoglobin as a Predictor for Type 2 Diabetes in Korean Adults (Korean Diabetes J 33(4):306-314, 2009)
- The Combination of Fasting Plasma Glucose and Glycosylated Hemoglobin as a Predictor for Type 2 Diabetes in Korean Adults (Korean Diabetes J 33(4):306-314, 2009)
- Postpartum glycosilated hemoglobin AIC and C - peptide levels in mother of macrosomia