Korean J Hematol.
2011 Jun;46(2):96-102. 10.5045/kjh.2011.46.2.96.
Treatment outcomes in children with Burkitt lymphoma and L3 acute lymphoblastic leukemia treated using the lymphoma malignancy B protocol at a single institution
- Affiliations
-
- 1Department of Pediatrics, Institute of Health Science, Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hsahn@snu.ac.kr
- KMID: 1782773
- DOI: http://doi.org/10.5045/kjh.2011.46.2.96
Abstract
- BACKGROUND
We compared the outcomes of patients with Burkitt lymphoma and French-American-British (FAB) L3 acute lymphoblastic leukemia treated using Lymphoma Malignancy B (LMB) or other treatment protocols.
METHODS
Thirty-eight patients diagnosed between July 1996 and December 2007 were treated using LMB 96, and 22 patients diagnosed between January 1991 and May 1998 (defined as the early period) were treated using the D-COMP or CCG-106B protocols. We retrospectively reviewed their medical records and analyzed cumulative survival according to the treatment period by using Kaplan-Meier analysis.
RESULTS
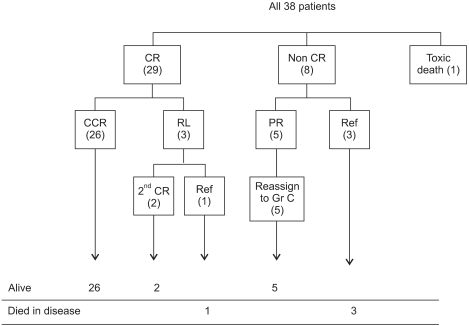
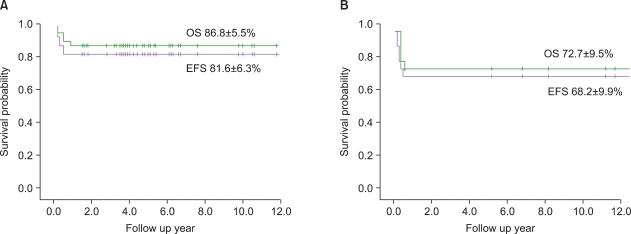
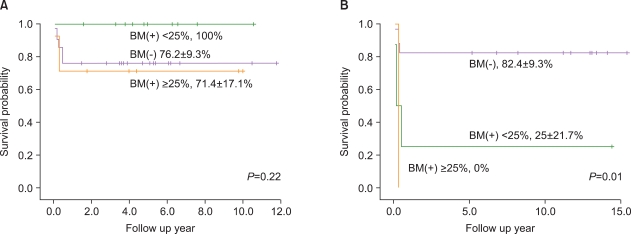
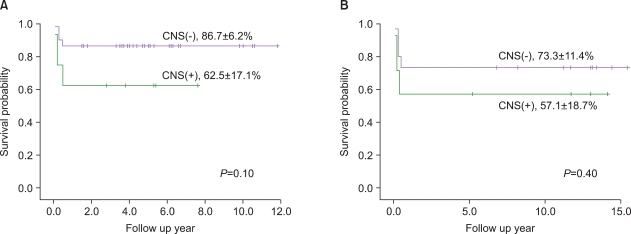
There were no intergroup differences in the distribution of age, disease stage, or risk group. The median follow-up period of the 33 live patients in the LMB group was 72 months (range, 36-170 months). Overall survival (OS) and event-free survival (EFS) of patients treated using LMB 96 were 86.8%+/-5.5% and 81.6%+/-6.3%, respectively, whereas OS and EFS of patients treated in the early period were 72.7%+/-9.6% and 68.2%+/-9.9%, respectively. In the LMB 96 group, OS of cases showing non-complete response (N=8) was 62.5%+/-17.1%, and OS of relapsed or primary refractory cases (N=6) was 33.3%+/-19.3%. Central nervous system (CNS) disease, high lactate dehydrogenase levels at diagnosis, and treatment response were significant prognostic factors.
CONCLUSION
Survival outcome has drastically improved over the last 2 decades with short-term, dose-intensive chemotherapy. However, CNS involvement or poor response to chemotherapy was worse prognostic factors; therefore, future studies addressing this therapeutic challenge are warranted.
MeSH Terms
Figure
Cited by 1 articles
-
The next step for Burkitt lymphoma
Bin Cho
Korean J Hematol. 2011;46(2):60-61. doi: 10.5045/kjh.2011.46.2.60.
Reference
-
1. Sandlund JT, Downing JR, Crist WM. Non-Hodgkin's lymphoma in childhood. N Engl J Med. 1996; 334:1238–1248. PMID: 8606720.
Article2. Hecht JL, Aster JC. Molecular biology of Burkitt's lymphoma. J Clin Oncol. 2000; 18:3707–3721. PMID: 11054444.
Article3. Patte C, Philip T, Rodary C, et al. Improved survival rate in children with stage III and IV B cell non-Hodgkin's lymphoma and leukemia using multi-agent chemotherapy: results of a study of 114 children from the French Pediatric Oncology Society. J Clin Oncol. 1986; 4:1219–1226. PMID: 3525767.
Article4. Patte C, Philip T, Rodary C, et al. High survival rate in advanced-stage B-cell lymphomas and leukemias without CNS involvement with a short intensive polychemotherapy: results from the French Pediatric Oncology Society of a randomized trial of 216 children. J Clin Oncol. 1991; 9:123–132. PMID: 1985161.
Article5. Gentet JC, Patte C, Quintana E, et al. Phase II study of cytarabine and etoposide in children with refractory or relapsed non-Hodgkin's lymphoma: a study of the French Society of Pediatric Oncology. J Clin Oncol. 1990; 8:661–665. PMID: 2313335.
Article6. Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007; 109:2736–2743. PMID: 17138821.
Article7. Park ES, Kim SD, Kang HJ, Choi HS, Shin HY, Ahn HS. Treatment of B-cell acute lymphoblastic leukemia and B-cell lymphoma. Korean J Pediatr Hematol Oncol. 2002; 9:166–176.8. Patte C, Auperin A, Michon J, et al. The Société Française d'Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001; 97:3370–3379. PMID: 11369626.9. Meadows AT, Sposto R, Jenkin RD, et al. Similar efficacy of 6 and 18 months of therapy with four drugs (COMP) for localized non-Hodgkin's lymphoma of children: a report from the Childrens Cancer Study Group. J Clin Oncol. 1989; 7:92–99. PMID: 2642543.
Article10. Gaynon PS, Steinherz PG, Bleyer WA, et al. Improved therapy for children with acute lymphoblastic leukemia and unfavorable presenting features: a follow-up report of the Childrens Cancer Group Study CCG-106. J Clin Oncol. 1993; 11:2234–2242. PMID: 8229139.
Article11. Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980; 7:332–339. PMID: 7414342.12. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966; 50:163–170. PMID: 5910392.13. Atra A, Gerrard M, Hobson R, Imeson JD, Hann IM, Pinkerton CR. Outcome of relapsed or refractory childhood B-cell acute lymphoblastic leukaemia and B-cell non-Hodgkin's lymphoma treated with the UKCCSG 9003/9002 protocols. Br J Haematol. 2001; 112:965–968. PMID: 11298592.
Article14. Attarbaschi A, Dworzak M, Steiner M, et al. Outcome of children with primary resistant or relapsed non-Hodgkin lymphoma and mature B-cell leukemia after intensive first-line treatment: a population-based analysis of the Austrian Cooperative Study Group. Pediatr Blood Cancer. 2005; 44:70–76. PMID: 15368550.
Article15. Fujita N, Mori T, Mitsui T, Inada H, Horibe K, Tsurusawa M. The role of hematopoietic stem cell transplantation with relapsed or primary refractory childhood B-cell non-Hodgkin lymphoma and mature B-cell leukemia: a retrospective analysis of enrolled cases in Japan. Pediatr Blood Cancer. 2008; 51:188–192. PMID: 18428432.
Article16. Won SC, Han JW, Kwon SY, et al. Autologous peripheral blood stem cell transplantation in children with non-Hodgkin's lymphoma: a report from the Korean society of pediatric hematology-oncology. Ann Hematol. 2006; 85:787–794. PMID: 16932891.
Article17. Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2009; 52:177–181. PMID: 18816698.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spontaneous Tumor Lysis Syndrome Presenting Acute Kidney Injury with Extreme Hyperuricemia and Urinary Stone: A Rare Case of Spontaneous Tumor Lysis Syndrome
- Treatment Outcome of Childhood B-cell Lymphoma and L3 Acute Lymphoblastic Leukemia from a Single Institution
- Therapy-related Acute Myeloid Leukemia Following Treatment for Burkitt's Lymphoma
- Burkitt's Lymphoma Developed Acute Leukemia: A report of two cases
- Treatment of Acute Lymphoblastic Leukemia in children