Infect Chemother.
2009 Jun;41(3):160-164. 10.3947/ic.2009.41.3.160.
A Study on the Adverse Events of the Yellow Fever Vaccine at an International Travelers' Clinic
- Affiliations
-
- 1Department of Pediatrics, National Medical Center, Seoul, Korea.
- 2Department of Internal Medicine, National Medical Center, Seoul, Korea.
- 3Department of International Travelers' Clinic, National Medical Center, Seoul, Korea.
- KMID: 1782318
- DOI: http://doi.org/10.3947/ic.2009.41.3.160
Abstract
-
BACKGROUND: Yellow fever (YF) can be prevented through vaccination, but YF vaccination causes adverse events. The increasing number of travelers to YF-endemic areas prompted an investigation of YF vaccination's adverse events on Koreans.
MATERIALS AND METHODS
From January to December 2007, 318 live-17DD vaccinees at the International Travelers' Clinic of the National Medical Center were enrolled in this study.
RESULTS
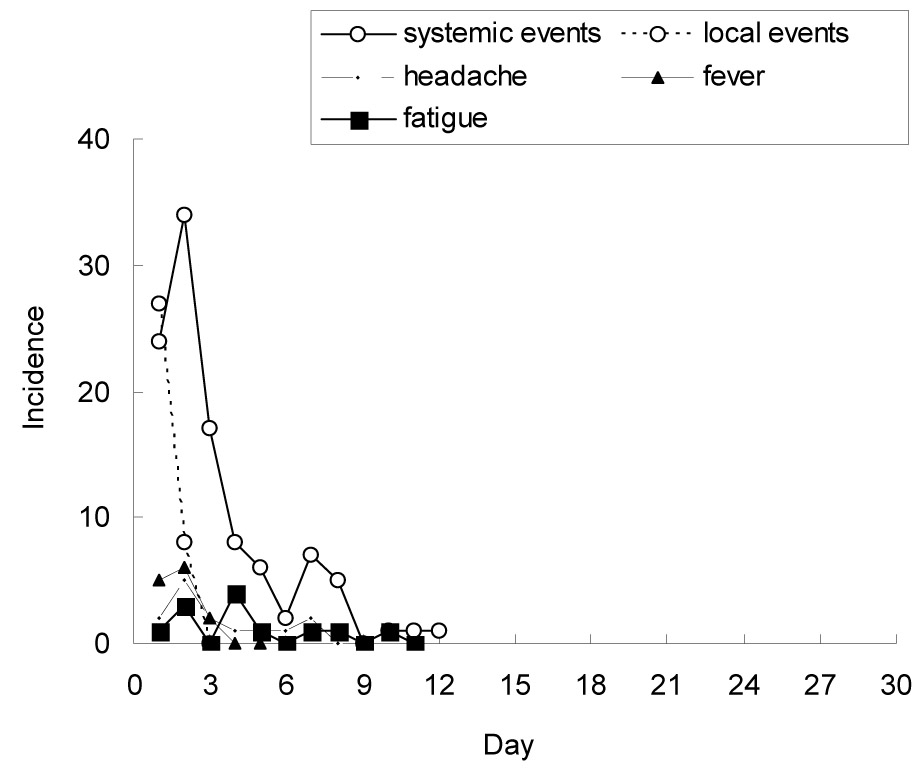
The adverse events were evaluated through six telephone interviews of 309 subjects (male: 168, 54.4%) on days 3, 6, 9, 16, 23, and 30 after the administration of the vaccine. There were 106 adverse events in 97 (31.4%) subjects aged 11 months to 70 years (male: 56, 18.1%). Of the 34 (11.0%) subjects who had underlying diseases, 3 (1.0%) reported adverse events (P=0.06). Nineteen (6.1%) of the 72 (23.3%) subjects who concurrently received other vaccines also experienced adverse events (P=0.29). Those who had underlying illnesses and those aged 10 to 19 years reported more frequent adverse events (P=0.06 and 0.14, respectively), but the significance of this finding is uncertain. Most of the adverse events occurred within 10 days after the vaccination and spontaneously subsided.
CONCLUSION
This study shows that most of the YF vaccine's adverse events are well tolerated and that the vaccine safely protects a vaccinee from YF.
Keyword
MeSH Terms
Figure
Reference
-
1. Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007. 52:209–229.
Article2. Center for Diseases Control Prevention (CDC). Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2002. 51(RR-17):1–10.3. Monath TP, Martin SC, Dirk ET. Plotkin SA, Orenstein WA, editors. Yellow fever. Vaccines. 2008. 5th ed. Philadelphia: WB Saunders;959–1055.
Article4. Marfin AA, Eidex RS, Kozarsky PE, Cetron MS. Yellow fever and Japanese encephalitis vaccines: indications and complications. Infect Dis Clin North Am. 2005. 19:151–168.
Article5. Barnett ED. Yellow fever : epidemiology and prevention. Clin Infect Dis. 2007. 44:850–856.6. Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001. 1:11–20.
Article7. Engel AR, Vasconcelos PF, McArthur MA, Barrett AD. Charaterization of a viscerotropic yellow fever vaccine variant from a patient in Brazil. Vaccine. 2006. 24:2803–2809.
Article8. Pugachev KV, Guirakhoo F, Monath TP. New developments in flavivirus vaccines with special at-tention to yellow fever. Curr Opin Infect Dis. 2005. 18:387–394.
Article9. Camacho LA, Freire Mda S, Leal Mda L, Aguiar SG, Nascimento JP, Iguchi T, Lozana Jde A, Farias RH. Collaborative group for the study of yellow fever vaccines. Immunogenicity of WHO-17D and brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica. 2004. 38:671–678.
Article10. Pugachev KV, Ocran SW, Guirakhoo F, Furby D, Monath TP. Heterogenous nature of the genome of the ARILVAX yellow fever 17D vaccine revealed by consensus sequencing. Vaccine. 2002. 25:996–999.11. Barrett AD, Monath TP, Barban V, Niedrig M, Teuwen DE. 17D yellow fever vaccines: new insights. A report of a workshop held during the World Congress on medicine and health in the tropics, Marseille, France, Monday 12 September 2005. Vaccine. 2007. 25:2758–2765.12. Monath TP, Cetron MS. Prevention of yellow fever in persons traveling to the tropics. Clin Infect Dis. 2002. 34:1369–1378.
Article13. Tomori O. Yellow fever: the recurring plague. Crit Rev Clin Lab Sci. 2004. 41:391–427.
Article14. Martin M, Weld LH, Tsai TF, Mootrey GT, Chen RT, Niu M, Cetron MS. GeoSentinel Yellow Fever Working Group. Advanced age as a risk factor for illness temporally associated with yellow fever vaccination. Emerg Infect Dis. 2001. 7:945–951.
Article15. Khromova AY, Eidex RB, Weld LH, Kohl KS, Bradshaw RD, Chen RT, Cetron MS. The Yellow Fever Vaccine Safety Working Group. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events. Vaccine. 2005. 23:3256–3263.
Article16. Monath TP, Cetron MS, McCarthy K, Nichols R, Archambault WT, Weld L, Bedford P. Yellow fever 17D vaccine safety and immunogenicity in the elderly. Hum Vaccin. 2005. 1:207–214.
Article17. Huntington MK. The traveling family. Prim Care. 2002. 29:1007–1025.
Article18. World Health Organiztion. Yellow fever situation in Africa and South America, 2005. Wkly Epidemiol Rec. 2006. 81:317–324.19. Choudri Y, Walop W. Review of adverse events reported following use of yellow fever vaccine-Canada, 1987-2000. Can Commun Dis Rep. 2002. 28:9–15.