J Korean Med Sci.
2006 Oct;21(5):954-957. 10.3346/jkms.2006.21.5.954.
A Case of Infantile Alexander Disease Accompanied by Infantile Spasms Diagnosed by DNA Analysis
- Affiliations
-
- 1Department of Pediatrics, Dongguk University College of Medicine, Gyeongju, Korea. pedepi@medimail.co.kr
- 2Department of Laboratory Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1781924
- DOI: http://doi.org/10.3346/jkms.2006.21.5.954
Abstract
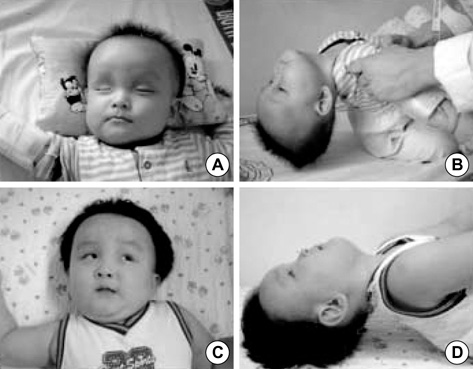
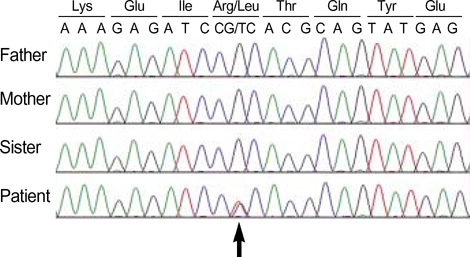
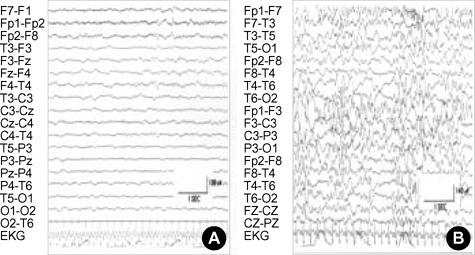
- Alexander disease (AD) is a rare leukodystrophy of the central nervous system of unknown etiology. AD is characterized by progressive failure of central myelination and the accumulation of Rosenthal fibers in astrocytes, and is inevitably lethal in nature. Symptomatically, AD is associated with leukoencephalopathy with macrocephaly, seizures, and psychomotor retardation in infants, and usually leads to death within the first decade. Its characteristic magnetic resonance imaging (MRI) findings have been described as demyelination predominantly in the frontal lobe. Moreover, dominant mutations in the GFAP gene, coding for glial fibrillary acidic protein (GFAP), a principal astrocytic intermediate filament protein, have been shown to lead to AD. The disease can now be detected by genetic diagnosis. We report the Korean case of an 8-month-old male patient with AD. He was clinically characterized due to the presence of psychomotor retardation, megalencephaly, spasticity, and recurrent seizures including infantile spasms which is a remarkable presentation. Demyelination in the frontal lobe and in a portion of the temporal lobe was demonstrated by brain MRI. Moreover, DNA analysis of peripheral blood showed the presence of a R239L mutation in the GFAP gene, involving the replacement of guanine with thymine.
Keyword
MeSH Terms
Figure
Reference
-
1. Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet. 2001. 27:117–120.
Article2. Alexander WS. Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain. 1949. 72:373–381.
Article3. Jeon HJ, Lee BH, Hwang SH, Hwang YS, Chi JG. A case of Alexander's disease. J Korean Child Neurol Soc. 1993. 1:173–178.4. Gordon N. Alexander's disease. Eur J Paediatr Neurol. 2003. 7:395–399.5. Johnson AB. Alexander's disease: a leukodystrophy caused by a mutation in GFAP. Neurochem Res. 2004. 29:961–964.6. Noetzel MJ. Diagnosing "undiagnosed" leukodystrophies: The role of molecular genetics. Neurology. 2004. 62:847–848.
Article7. Russo LS, Aron A, Anderson PJ. Alexander's disease: A report and reappraisal. Neurology. 1976. 26:607–614.
Article8. Townsend JJ, Wilson JF, Harris T, Coulter D, Fife R. Alexander's disease. Acta Neuropathol. 1985. 67:163–166.
Article9. Neal JW, Cave EM, Singhrao SK, Cole G, Wallace SJ. Alexander's disease in infancy and childhood: A case report of two cases. Acta Neuropathol. 1992. 84:322–327.
Article10. Deprez M, D'Hooghe M, Mission JP, de Leval L, Ceuterick C, Reznik M, Martin JJ. Infantile and juvenile presentation of Alexander's disease: A report of two cases. Acta Neurol Scand. 1999. 99:158–165.11. Hanefeld FA. Alexander disease: past and present. Cell Mol Life Sci. 2004. 61:2750–2752.
Article12. Wakabayashi K, Lai M, Masuko K, Yamashita S, Yamada M, Iwamoto H, Aida N, Shiroma N, Kanazawa N, Tsujino S. A case of long-term survival of a patient with infantile Alexander's disease diagnosed by DNA analysis. No To Hattatsu. 2005. 37:55–59.13. Pridmore CL, Baraitser M, Harding B, Boyd SG, Kendall B, Brett EM. Alexander's disease: clues to diagnosis. J Child Neurol. 1993. 8:134–144.
Article14. Gingold MK, Bodensteiner JB, Schochet SS, Jaynes M. Alexander's disease: unique presentation. J Child Neurol. 1999. 14:325–329.
Article15. Goldman JE, Corbin E. Isolation of a major protein component of Rosenthal fibers. Am J Pathol. 1988. 130:569–578.16. Iwaki T, Iwaki A, Tateishi J, Sakaki Y, Goldman JE. Alpha B-crystallin and 27-kd heat shock protein are regulated by stress conditions in the central nervous system and accumulate in Rosenthal fibers. Am J Pathol. 1993. 143:487–495.17. Arend AO, Leary PM, Rutherfoord GS. Alexander's disease: a case report with brain biopsy, ultrasound, CT scan and MRI finding. Clin Neuropathol. 1991. 10:122–126.18. van der Knaap MS, Naidu S, Breiter SN, Blaser S, Stroink H, Springer S, Begeer JC, van Coster R, Barth PG, Thomas NH, Valk J, Powers JM. Alexander disease: Diagnosis with MR imaging. Am J Neuroradiol. 2001. 22:541–552.19. Li R, Messing A, Goldman JE, Brenner M. GFAP mutations in Alexander disease. Int J Dev Neurosci. 2002. 20:259–268.
Article20. Wohlwill FJ, Bernstein J, Yakovlev PI. Dysmyelinogenic leukodystrophy; report of a case of a new, presumably familial type of leukodystrophy with megalobarencephaly. J Neuropathol Exper Neurol. 1959. 18:359–383.21. Schwankhaus JD, Parisi JE, Gulledge WR, Chin L, Currier RD. Hereditary adult onset Alexander's disease with palatal myoclonus, spastic paraparesis, and cerebellar ataxia. Neurology. 1995. 45:2266–2271.