J Korean Med Sci.
2006 Oct;21(5):932-935. 10.3346/jkms.2006.21.5.932.
A Case of Imported Plasmodium ovale malaria
- Affiliations
-
- 1Department of Laboratory Medicine, Inje University Sanggye Paik Hospital, Korea. kscosby@sanggyepaik.ac.kr
- 2Department of Internal Medicine, Inje University Sanggye Paik Hospital, Korea.
- 3Department of Biomedical Laboratory Science, College of Biomedical Science and Engineering, Inje University, Seoul, Korea.
- KMID: 1781918
- DOI: http://doi.org/10.3346/jkms.2006.21.5.932
Abstract
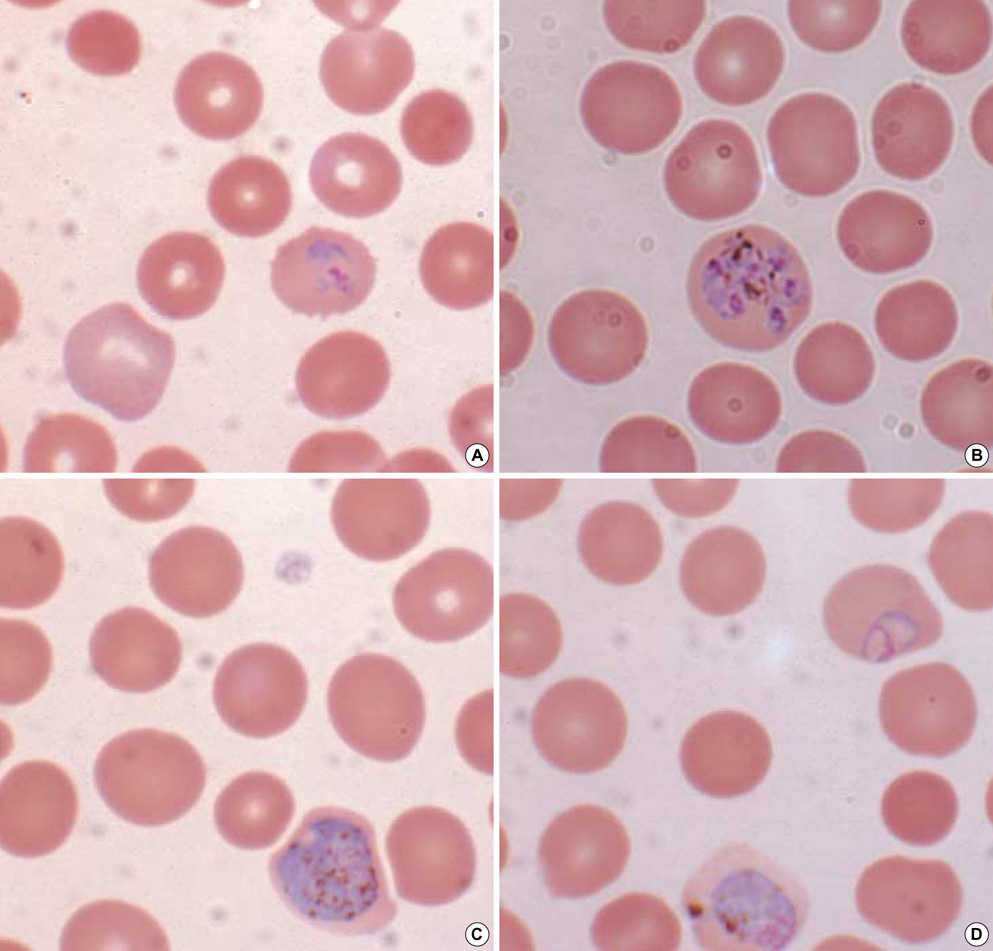
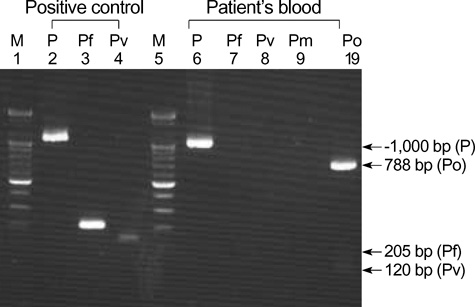
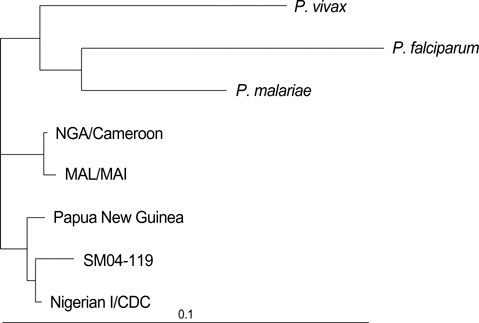
- There have been reports in Korea of imported malaria cases of four Plasmodium species, but there has been no report of imported Plasmodium ovale malaria confirmed by molecular biological methods. We report an imported case of that was confirmed by Wright-Giemsa-stained peripheral blood smear and nested polymerase chain reaction targeting the small subunit ribosomal RNA gene. The amplified DNA was sequenced and compared with other registered P. ovale isolates. The isolate in this study was a member of the classic type group. The patient was a 44-yr-old male who had worked as a woodcutter in Cote d'Ivoire in tropical West Africa. He was treated with hydroxychloroquine and primaquine and discharged following improvement. In conclusion, P. ovale should be considered as an etiology in the imported malaria in Korea, because the number of travelers to P. ovale endemic regions has recently increased.
Keyword
MeSH Terms
Figure
Reference
-
1. Win TT, Jalloh A, Tantular IS, Tsuboi T, Ferreira MU, Kimura M, Kawamoto F. Molecular analysis of Plasmodium ovale variants. Emerg Infect Dis. 2004. 10:1235–1240.2. Win TT, Lin K, Mizuno S, Zhou M, Liu Q, Ferreira MU, Tantular IS, Kojima S, Ishii A, Kawamoto F. Wide distribution of Plasmodium ovale in Myanmar. Trop Med Int Health. 2002. 7:231–239.3. Kawamoto F, Liu Q, Ferreira MU, Tantular IS. How prevalent are Plasmodium ovale and P. malariae in East Asia? Parasitol Today. 1999. 15:422–426.4. Faye FB, Spiegel A, Tall A, Sokhna C, Fontenille D, Rogier C, Trape JF. Diagnostic criteria and risk factors for Plasmodium ovale malaria. J Infect Dis. 2002. 186:690–695.5. Mehlotra RK, Lorry K, Kastens W, Miller SM, Alpers MP, Bockarie M, Kazura JW, Zimmerman PA. Random distribution of mixed-species malaria infections in Papua New Guinea. Am J Trop Med Hyg. 2000. 62:225–231.6. Lee SY, Ko TS, Chi HS, Park YS. Four cases of the imported falciparum malaria in children. J Korean Pediatr Soc. 1997. 40:249–254.7. Shin KS, Kim JS, Ryu SW, Suh IB, Lim CS. A case of Plasmodium vivax infection diagnosed after treatment of imported falciparum malaria. Korean J Clin Pathol. 2001. 21:360–364.8. Soh CT, Lee KT, Im KI, Min DY, Ahn MH, Kim JJ, Yong TS. Current status of malaria in Korea. Yonsei Rep Trop Med. 1985. 16:11–18.9. Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993. 61:315–320.
Article10. Warhurst DC, Williams JE. Laboratory diagnosis of malaria. J Clin Pathol. 1996. 49:533–538.11. Richie TL. Interactions between malaria parasites infecting the same vertebrate host. Parasitology. 1988. 96:607–639.
Article12. May J, Mockenhaupt FP, Ademowo OG, Falusi AG, Olumese PE, Bienzle U, Meyer CG. High rate of mixed and subpatent malarial infections in southwest Nigeria. Am J Trop Med Hyg. 1999. 61:339–343.
Article13. Qari SH, Shi YP, Pieniazek NJ, Collins WE, Lal AA. Phylogenetic relationship among the malaria parasites based on small subunit rRNA gene sequences: monophyletic nature of the human malaria parasite, Plasmodium falciparum. Mol Phylogenet Evol. 1996. 6:157–165.14. Nzeyimana I, Henry MC, Dossou-Yovo J, Doannio JM, Diawara L, Carnevale P. The epidemiology of malaria in the southwestern forests of the Ivory Coast (Tai region). Bull Soc Pathol Exot. 2002. 95:89–94.15. Rubio JM, Post RJ, van Leeuwen WM, Henry MC, Lindergard G, Hommel M. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: the semi-nested multiplex malaria PCR (SnM-PCR). Trans R Soc Trop Med Hyg. 2002. 96:199–204.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Molecular Analysis on Imported Plasmodium ovale curtisi and P. ovale wallikeri Malaria Cases from West and South Africa during 2013-2016
- A Case of Plasmodium ovale Malaria Imported from West Africa
- A Case of Plasmodium ovale Malaria Imported from West Africa
- Characteristics of Imported Malaria and Species of Plasmodium Involved in Shandong Province, China (2012-2014)
- A Case of Plasmodium ovale wallikeri Infection in a Chinese Worker Returning from West Africa