Evaluation of Anti-Malarial Effects of Mass Chemoprophylaxis in the Republic of Korea Army
- Affiliations
-
- 1Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Occupational Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Korean Armed Forces, Daegu Hospital, Daegu, Korea.
- 4Department of Surgery, Kyunghee University College of Medicine, Seoul, Korea.
- 5Department of Emergency Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Pathology, Hanyang University College of Medicine, Seoul, Korea.
- 7Department of Microbiology, Gachon Medical School, Incheon, Korea. seorak@dreamwiz.com
- KMID: 1781749
- DOI: http://doi.org/10.3346/jkms.2005.20.5.707
Abstract
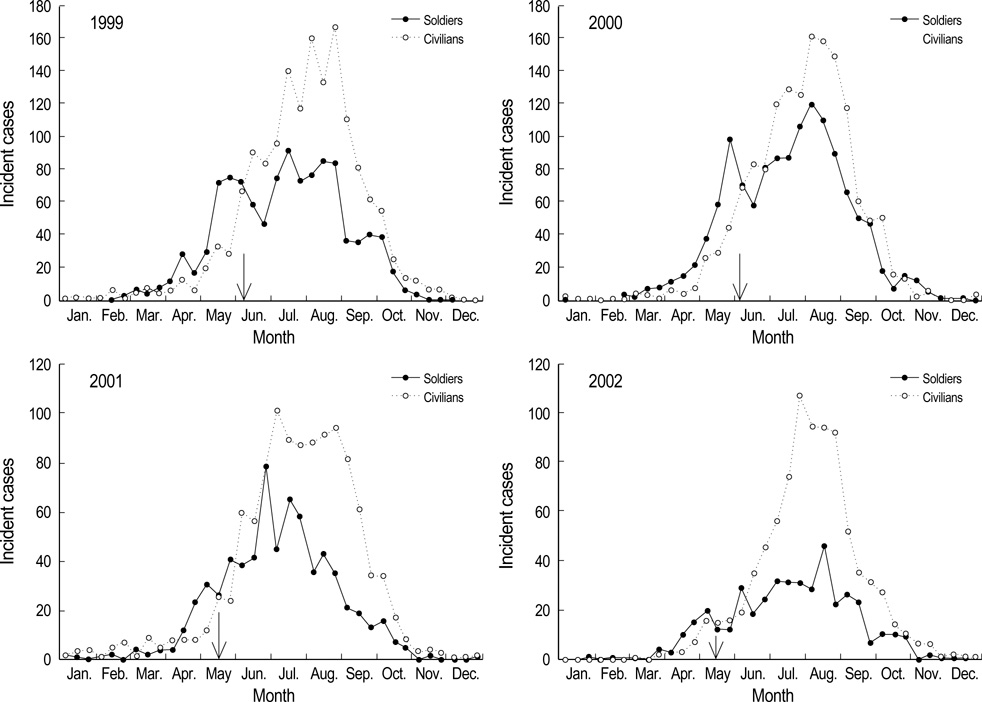
- Vivax malaria was endemic on the Korean peninsula for many centuries until the late 1970's when the Republic of Korea (ROK) was declared "malaria free". Since its re-emergence in 1993, the number of malaria cases in the military increased exponentially through 2000 near the demilitarized zone. Chemoprophylaxis with chloroquine and primaquine has been used in the ROK Army since 1997 in an attempt to reduce the number of the malaria cases throughout the ROK. Data show that chemoprophylaxis contributed, in part, to the decrease in the number of malaria cases among military personnel. However, mass chemoprophylaxis on a large scale in the ROK Army is unprecedented and extensive supervision and monitoring is warranted to determine its effectiveness and to monitor the appearance of chloroquine tolerant/resistant strains of Plasmodium vivax.
Keyword
MeSH Terms
-
Antimalarials/therapeutic use
Chemoprevention/methods/statistics and numerical data
Chloroquine/*therapeutic use
Disease Outbreaks/*prevention and control/*statistics and numerical data
Humans
Incidence
Korea/epidemiology
Malaria, Vivax/*epidemiology/*prevention and control
Military Personnel/*statistics and numerical data
Outcome Assessment (Health Care)
Prevalence
Primaquine/*therapeutic use
Research Support, Non-U.S. Gov't
Risk Assessment/methods
Risk Factors
Treatment Outcome
Figure
Cited by 8 articles
-
Serial follow-up of malaria-induced splenic infarction: A case report
Sung Hyun Kim, Hong Sung Jung, Sejin Park
Ann Hepatobiliary Pancreat Surg. 2020;24(2):239-242. doi: 10.14701/ahbps.2020.24.2.239.Cost-Benefit Analysis of Malaria Chemoprophylaxis and Early Diagnosis for Korean Soldiers in Malaria Risk Regions
Hee-sung Kim, Gilwon Kang, Sunmi Lee, Chang-gyo Yoon, Minyoung Kim
J Korean Med Sci. 2018;33(10):. doi: 10.3346/jkms.2018.33.e59.Status of Vivax Malaria after Re-emergence in South Korea
Joon-Sup Yeom, Jae-Won Park
Infect Chemother. 2008;40(4):191-198. doi: 10.3947/ic.2008.40.4.191.Evaluation of the Current Status of Malaria Elimination Project in the Republic of Korea and Suggestion for Improvement of Its Efficacy
Jae-Won Park, Jee-Young Hong, Joon-Sup Yeom, Sung-Rae Cho, Dae-Kyu Oh
Infect Chemother. 2009;41(1):42-53. doi: 10.3947/ic.2009.41.1.42.Commencement of the Meningococcal Vaccination for the Republic of Korea Army
Sang-Oh Lee
Infect Chemother. 2013;45(1):113-115. doi: 10.3947/ic.2013.45.1.113.Reemergence of Malaria in Korea
Weon-Gyu Kho
J Korean Med Assoc. 2007;50(11):959-966. doi: 10.5124/jkma.2007.50.11.959.Domestic and international trend of vector-borne disease
Hyun Jung Bahk, Dong Han Lee
J Korean Med Assoc. 2017;60(6):451-457. doi: 10.5124/jkma.2017.60.6.451.Status of Plasmodium vivax Malaria in the Republic of Korea after Reemergence
Jae-Won Park
Hanyang Med Rev. 2010;30(3):176-186. doi: 10.7599/hmr.2010.30.3.176.
Reference
-
1. Galinski MR, Barnwell JW. Plasmodium vivax: merozoites invasion of reticulocytes and considerations for malaria vaccine development. Parasitol Today. 1996. 12:20–29.
Article2. Paik YH, Ree HI, Shim JC. Malaria in Korea. Jpn J Exp Med. 1988. 58:55–56.3. Ree HI. Unstable vivax malaria in Korea. Korean J Parasitol. 2000. 38:119–138.
Article4. World Health Organisation. Synopsis of the world malaria situation in 1979. Wkly Epidemiol Rec. 1981. 56:145–149.5. Hankey DD, Jones R Jr, Coatney GR, Alving AS, Coker WG, Garrison PL, Donovan WN. Korean vivax malaria. I. Natural history and response to chloroquine. Am J Trop Med Hyg. 1953. 2:958–969.6. Coatney GR, Alving AS, Jones R Jr, Hankey DD, Robinson DH, Garrison PL, Coker WG, Donovan WN, Di Lorenzo A, Marx RL, Simmons IH. Korean vivax malaria. V. Cure of the infection by primaquine administered during long-term latency. Am J Trop Med Hyg. 1953. 2:985–988.7. Jones R, Jackson LS, Di Lorenzo A, Marx RL, Levy BL, Kenny EC, Gilbert M, Johnston MN, Alving AS. Korean vivax malaria. III. Curative effect and toxicity of primaquine in doses from 10 to 30 mg daily. Am J Trop Med Hyg. 1953. 2:977–982.8. Lee JS, Lee WJ, Cho SH, Ree HI. Outbreak of vivax malaria in areas adjacent to the demilitarized zone, South Korea, 1998. Am J Trop Med Hyg. 2002. 66:13–17.
Article9. Soh CT, Lee KT, Im KI, Min DY, Ahn MH, Kim JJ, Yong TS. Current status of malaria in Korea. Yonsei Rep Trop Med. 1985. 16:11–18.10. Feighner BH, Park SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the Republic of Korea. Emerg Infect Dis. 1998. 4:295–297.
Article11. National Institute of Health. Communicable Diseases Monthly Report, Korea. 1995-2002. Seoul: Korean National Institute of Health;(in Korean).12. Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, Moon SH, Kim TS, Chai JY, Oh MD, Choe KW. Vivax malaria: a continuing health threat to the Republic of Korea. Am J Trop Med Hyg. 2003. 69:159–167.
Article13. Ryu SH, Lee WJ, Kim YA, Chai JY, Park JW. Status of vivax malaria in the Republic of Korea in 2001. Korean J Infect Dis. 2002. 34:267–275.14. Yeom JS, Lee WJ, Ryu SH, Kim TS, Kim YA, Ahn SY, Yang HY, Park JW. Status of vivax malaria in the Republic of Korea in 2002. Infect Chemother. 2003. 35:385–392.15. Shute PG, Lupascu G, Branzei P, Maryon M, Constantinescu P, Bruce-Chwatt LJ, Draper CC, Killick-Kendrick R, Garnham PC. A strain of Plasmodium vivax characterized by prolonged incubation: the effect of numbers of sporozoites on the length of the prepatent period. Trans R Soc Trop Med Hyg. 1977. 70:474–481.
Article16. Ree HI. Can malaria be endemic in South Korea? Korean J Infect Dis. 1998. 30:397–400.17. Gilles HM, Warrell DA. Gilles HM, Warrell DA, editors. The Anopheles Vector. Bruce-Chwatt's Essential Malariology. 1993. 3rd ed. Boston: Edward Arnold Press;127–131.18. Centers for Disease Control and Prevention. Health information for international travel: 2003-2004. 2003. Atlanta: US Department of Health and Human Sevices.19. Committee to Advise on Tropical Medicine and Travel (CATMAT). Laboratory for Disease Control. Canadian recommendations for the prevention and treatment of malaria among international travelers. Can Commun Dis Rep. 2000. 26:Suppl 2. 1–42.20. Kain KC, Shanks GD, Keystone JS. Malaria chemoprophylaxis in the age of drug resistance. I. Currently recommended drug regimens. Clin Infect Dis. 2001. 33:226–234.
Article21. Shanks GD, Kain KC, Keystone JS. Malaria chemoprophylaxis in the age of drug resistance. II. Drugs that may be available in the future. Clin Infect Dis. 2001. 33:381–385.
Article22. WHO. International travel and health: vaccination requirements and health advice 2000. 2000. Geneva: World Health Organization.23. Touze JE, Paule P, Baudon D, Boutin JP. Malaria prophylaxis in the French Armed Forces: evolution of concepts. Med Trop (Mars). 2001. 61:79–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cost-Benefit Analysis of Malaria Chemoprophylaxis and Early Diagnosis for Korean Soldiers in Malaria Risk Regions
- Studies on the vivax malaria readmitted in military hospital
- Two Cases of Malarial Infection in Pregnancy
- An Outbreak of Vivax Malaria in Republic of Korea in 1999
- Case of Malarial Hepatitis by Plasmodium Vivax