Serial Interferon-gamma Release Assays for the Diagnosis of Latent Tuberculosis Infection in Patients Treated with Immunosuppressive Agents
- Affiliations
-
- 1Department of Laboratory Medicine, Dong-A University College of Medicine, Busan, Korea. progreen@dau.ac.kr
- 2Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Preventive Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 1781691
- DOI: http://doi.org/10.3343/kjlm.2011.31.4.271
Abstract
- BACKGROUND
We assessed the efficacy of serial interferon-gamma release assays (IGRAs) for the diagnosis of latent tuberculosis infection (LTBI) in patients receiving immunosuppressive agents for treatment of rheumatic diseases in Korea.
METHODS
Of 276 patients who underwent consecutive screening with one of two IGRAs [QuantiFERON-TB Gold or QuantiFERON-TB Gold In-Tube], 66 patients were evaluated by the serial IGRA for detection of LTBI during therapy with immunosuppressive agents. Information on clinical diagnosis, medication, previous TB, blood cell count, tuberculin skin test, and interferon-gamma (IFN-gamma) level measured by IGRA was collected.
RESULTS
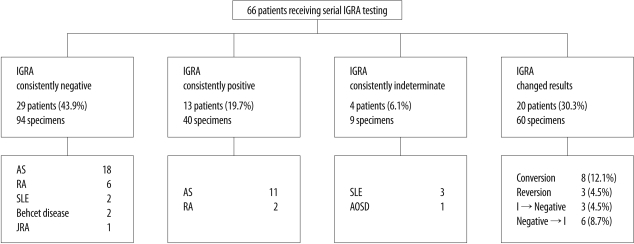
Of the 66 patients, the initial IGRA was positive in 24.2%, negative in 65.2%, and indeterminate in 10.6%. Forty-six patients (69.7%) showed consistent IGRA results during follow-up, and 13 patients (19.7%) had consistently positive results. IGRA conversion rate was 12.1% (8/66) and reversion rate was 4.5% (3/66). Conversion of IGRA results was only observed in ankylosing spondylitis patients, and the median interval between the two tests in patients with conversion was 8.5 months. The mean IFN-gamma level in the group of patients with consistently positive IGRA results was higher than that in the group with inconsistently positive results, although this trend was not statistically significant (P=0.293). Indeterminate results were observed most frequently in patients with systemic lupus erythematosus.
CONCLUSIONS
In patients receiving immunosuppressive agents, both IGRA conversions and reversions were observed. Serial IGRA testing may not be needed in patients with a positive initial IGRA result showing high IFN-gamma levels, because of high consistency in the test results.
Keyword
MeSH Terms
-
Adult
Blood Cell Count
Female
Follow-Up Studies
Humans
Immunosuppressive Agents/*therapeutic use
Interferon-gamma/*analysis
*Interferon-gamma Release Tests
Latent Tuberculosis/complications/*diagnosis/metabolism
Lupus Erythematosus, Systemic/complications/diagnosis/metabolism
Male
Middle Aged
Rheumatic Diseases/complications/diagnosis/drug therapy
Spondylitis, Ankylosing/complications/diagnosis/metabolism
Tuberculin Test
Figure
Cited by 4 articles
-
Clinical Applications of Interferon-gamma Release Assays
Kwang-Sook Woo, Kyeong-Hee Kim
Lab Med Online. 2016;6(1):8-11. doi: 10.3343/lmo.2016.6.1.8.Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management
Dong Il Park, Tadakazu Hisamatsu, Minhu Chen, Siew Chien Ng, Choon Jin Ooi, Shu Chen Wei, Rupa Banerjee, Ida Normiha Hilmi, Yoon Tae Jeen, Dong Soo Han, Hyo Jong Kim, Zhihua Ran, Kaichun Wu, Jiaming Qian, Pin-Jin Hu, Katsuyoshi Matsuoka, Akira Andoh, Yasuo Suzuki, Kentaro Sugano, Mamoru Watanabe, Toshifumi Hibi, Amarender S. Puri, Suk-Kyun Yang
Intest Res. 2018;16(1):17-25. doi: 10.5217/ir.2018.16.1.17.High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India
Ashish Agarwal, Saurabh Kedia, Saransh Jain, Vipin Gupta, Sawan Bopanna, Dawesh P Yadav, Sandeep Goyal, Venigalla Pratap Mouli, Rajan Dhingra, Govind Makharia, Vineet Ahuja
Intest Res. 2018;16(4):588-598. doi: 10.5217/ir.2018.00023.Diagnosis and Treatment of Latent Tuberculosis Infection in Patients with Inflammatory Bowel Diseases due to Initiation of Anti-Tumor Necrosis Factor Therapy
Tae Sun Shim
Intest Res. 2014;12(1):12-19. doi: 10.5217/ir.2014.12.1.12.
Reference
-
1. Small PM, Fujiwara PI. Management of tuberculosis in the United States. N Engl J Med. 2001; 345:189–200. PMID: 11463015.
Article2. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004; 350:2060–2067. PMID: 15141044.
Article3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med. 2002; 347:1860–1866. PMID: 12466511.4. Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002; 57:804–809. PMID: 12200526.5. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005; 293:2756–2761. PMID: 15941805.6. U.S. Food and Drug Administration. T-SPOT-TB-P070006. Updated on Jul 2008. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P070006.7. Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB gold test for detecting mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005; 54:49–55. PMID: 16357824.8. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010; 59:1–25. PMID: 20577159.9. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001; 345:1098–1104. PMID: 11596589.10. Korea Food and Drug Administration. Guideline for diagnosis and treatment of latent tuberculosis infection in patients treated with TNF blockers. 2004. Korea Food and Drug Administration.11. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008; 149:177–184. PMID: 18593687.
Article12. Park JH, Seo GY, Lee JS, Kim TH, Yoo DH. Positive conversion of tuberculin skin test and performance of interferon release assay to detect hidden tuberculosis infection during anti-tumor necrosis factor agent trial. J Rheumatol. 2009; 36:2158–2163. PMID: 19723901.
Article13. Chen DY, Shen GH, Hsieh TY, Hsieh CW, Lan JL. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum. 2008; 59:800–806. PMID: 18512714.
Article14. Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB gold in-tube assay. Clin Vaccine Immunol. 2008; 15:425–432. PMID: 18199741.
Article15. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005; 20:913–925. PMID: 16361797.
Article16. Hamdi H, Mariette X, Godot V, Weldingh K, Hamid AM, Prejean MV, et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res Ther. 2006; 8:R114. PMID: 16859506.17. Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008; 67:84–90. PMID: 17644549.18. Inanc N, Aydin SZ, Karakurt S, Atagunduz P, Yavuz S, Direskeneli H. Agreement between Quantiferon-TB gold test and tuberculin skin test in the identification of latent tuberculosis infection in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2009; 36:2675–2681. PMID: 19918035.
Article19. Malaviya AN, Kapoor S, Garg S, Rawat R, Shankar S, Nagpal S, et al. Preventing tuberculosis flare in patients with inflammatory rheumatic diseases receiving tumor necrosis factor-alpha inhibitors in India -- An audit report. J Rheumatol. 2009; 36:1414–1420. PMID: 19487263.20. Shovman O, Anouk M, Vinnitsky N, Arad U, Paran D, Litinsky I, et al. QuantiFERON-TB gold in the identification of latent tuberculosis infection in rheumatoid arthritis: a pilot study. Int J Tuberc Lung Dis. 2009; 13:1427–1432. PMID: 19861018.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Applications of Interferon-gamma Release Assays
- Diagnosis and treatment of latent tuberculosis infection
- Diagnosis and treatment of latent tuberculosis infection
- Diagnosis and Treatment of Latent Tuberculosis Infection
- Diagnosis and Treatment of Latent Tuberculosis Infection due to Initiation of Anti-TNF Therapy