Korean J Lab Med.
2010 Dec;30(6):654-659. 10.3343/kjlm.2010.30.6.654.
Evaluation of ARCHITECT HCV Core Antigen Assay
- Affiliations
-
- 1Department of Laboratory Medicine, Pusan National University School of Medicine, Pusan National University Hospital, Busan, Korea. hhkim@pusan.ac.kr
- 2Medical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 1781673
- DOI: http://doi.org/10.3343/kjlm.2010.30.6.654
Abstract
- BACKGROUND
Hepatitis C virus (HCV) core antigen (Ag) levels are known to be well correlating with HCV RNA levels, and may be used as an alternative marker of HCV replication for monitoring the response to HCV treatment. However, the low sensitivity of HCV core Ag assay has been an obstacle for clinical use. In this study, recently developed ARCHITECT HCV Ag assay (Abbott Laboratories, USA) was evaluated for analytical performance and clinical usefulness.
METHODS
A total of 109 sera from HCV infected patients including various genotypes of HCV (1b, 2, 2a/2c, 2b, and 3a) and 20 sera from healthy donors were used for evaluating the sensitivity, precision, and linearity of the HCV core Ag assay. The cross reactivity with HIV, hepatitis B virus and myeloma proteins (N=5, each) and correlation with HCV RNA PCR assay were also evaluated.
RESULTS
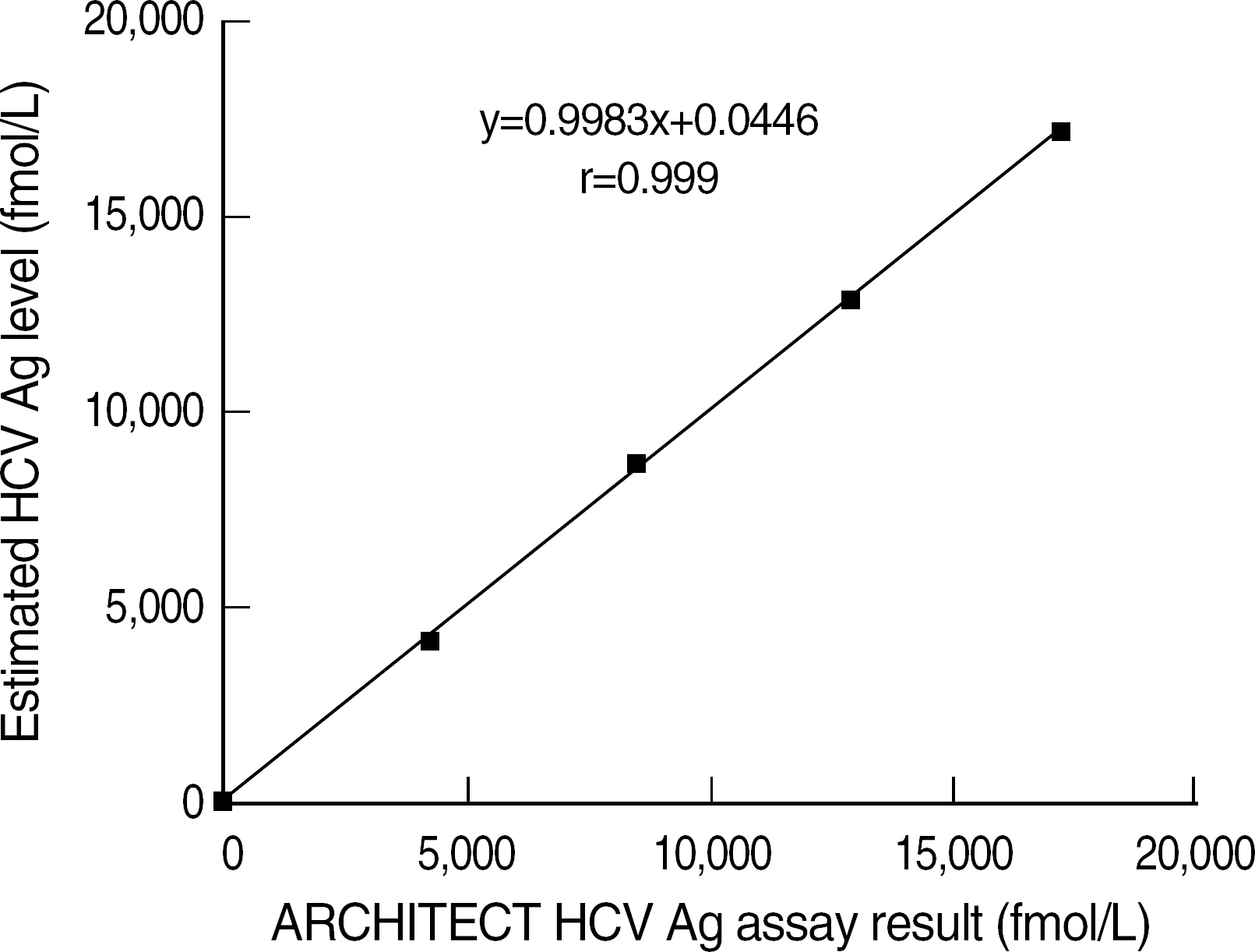
The sensitivity of the HCV core Ag assay was 97.2% (106/109) and there were no false positive results and cross reactivity. The within-run, between-run and between-day CVs were 3.0%, 2.5% and 3.0%, respectively. The levels of HCV core antigen showed a good correlation with those of HCV RNA quantification (r=0.940). The HCV Ag assay showed an excellent linearity in the range from 0.63 to 17,114 fmol/L (r=0.999).
CONCLUSIONS
The ARCHITECT HCV Ag assay was good in sensitivity, precision, and linearity and its results well correlated with HCV RNA levels. This assay could be used as a good marker of viral replication for monitoring the therapy response in chronically HCV infected patients.
Keyword
MeSH Terms
Figure
Reference
-
1.Alter HJ., Purcell RH., Shih JW., Melpolder JC., Houghton M., Choo QL, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989. 321:1494–500.
Article2.Kiyosawa K., Sodeyama T., Tanaka E., Gibo Y., Yoshizawa K., Nakano Y, et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990. 12:671–5.
Article3.Seeff LB., Hollinger FB., Alter HJ., Wright EC., Cain CM., Buskell ZJ, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001. 33:455–63.
Article4.Richter SS. Laboratory assays for diagnosis and management of hepatitis C virus infection. J Clin Microbiol. 2002. 40:4407–12.
Article5.Glynn SA., Wright DJ., Kleinman SH., Hirschkorn D., Tu Y., Heldebrant C, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005. 45:994–1002.
Article6.Pawlotsky JM. Clinical virology of hepatitis C. Marcellin P, editor. Management of patients with viral hepatitis. Paris: APMAHV;2004. p. 21–34.7.Simmonds P. Viral heterogeneity of the hepatitis C virus. J Hepatol. 1999. 31(S1):54–60.
Article8.Mondelli MU. Monitoring response to antiviral treatment by serum hepatitis C virus core antigen: too early to take shortcuts? J Hepatol. 2004. 40:536–8.
Article9.Takahashi M., Saito H., Higashimoto M., Atsukawa K., Ishii H. Benefit of hepatitis C virus core antigen assay in prediction of therapeutic response to interferon and ribavirin combination therapy. J Clin Microbiol. 2005. 43:186–91.
Article10.Zanetti AR., Romano L., Brunetto M., Colombo M., Bellati G., Tackney C. Total HCV core antigen assay: a new marker of hepatitis C viremia for monitoring the progress of therapy. J Med Virol. 2003. 70:27–30.
Article11.Tanaka E., Kiyosawa K., Matsumoto A., Kashiwakuma T., Hasegawa A., Mori H, et al. Serum levels of hepatitis C virus core protein in patients with chronic hepatitis C treated with interferon alfa. Hepatology. 1996. 23:1330–3.
Article12.González V., Padilla E., Diago M., Giménez MD., Solà R., Matas L, et al. Clinical usefulness of total hepatitis C virus core antigen quantification to monitor the response to treatment with peginterferon alpha-2a plus ribavirin∗. J Viral Hepat. 2005. 12:481–7.
Article13.National Committee for Clinical Laboratory Standards. Evaluation of the precision performance of clinical chemistry devices: approved guideline (EP5-A2). 2nd ed.Wayne, PA: NCCLS;2004.14.National Committee for Clinical Laboratory Standards. Evaluation of the linearity of quantitative measurement procedures: a statistical approach: approved guideline (EP6-A). Wayne, PA: NCCLS;2003.15.Morota K., Fujinami R., Kinukawa H., Machida T., Ohno K., Saegusa H, et al. A new sensitive and automated chemiluminescent micro-particle immunoassay for quantitative determination of hepatitis C virus core antigen. J Virol Methods. 2009. 157:8–14.
Article16.Seme K., Poljak M., Babic DZ., Mocilnik T., Vince A. The role of core antigen detection in management of hepatitis C: a critical review. J Clin Virol. 2005. 32:92–101.
Article17.Lee SY., Huh JW., Lee MA., Chung WS. Usefulness of HCV core protein for detection of HCV viremia. Korean J Clin Pathol. 2002. 22:114–8. (이수연, 허정원, 이미애, 정화순. C형 간염 바이러스 혈증 검출을 위한HCV core 단백검사의유용성. 대한임상병리학회지 2002;22:114-8.).18.Couroucé AM., Le Marrec N., Bouchardeau F., Razer A., Maniez M., Laperche S, et al. Efficacy of HCV core antigen detection during the preseroconversion period. Transfusion. 2000. 40:1198–202.
Article19.Tanaka T., Lau JY., Mizokami M., Orito E., Tanaka E., Kiyosawa K, et al. Simple fluorescent enzyme immunoassay for detection and quantification of hepatitis C viremia. J Hepatol. 1995. 23:742–5.
Article20.Kurtz JB., Boxall E., Qusir N., Shirley J., Coleman D., Chandler C. The diagnostic significance of an assay for ‘total’ hepatitis C core antigen. J Virol Methods. 2001. 96:127–32.
Article21.Bouvier-Alias M., Patel K., Dahari H., Beaucourt S., Larderie P., Blatt L, et al. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology. 2002. 36:211–8.
Article22.Yang SJ., Shin MG., Kim SH., Cho D., Kee SJ., Shin JH, et al. Usefulness of Track-C (total HCV core antigen) assays in anti-HCV positive patients. Korean J Lab Med. 2004. 24:244–9. (양성진, 신명근, 김수현,조덕, 기승정, 신종희등. Anti-HCV 양성환자에서 Track-C (total HCVcore antigen) 검사의유용성. 대한진단검사의학회지 2004;24:244-9.).23.Park Y., Lee JH., Kim BS., Kim do Y., Han KH., Kim HS. New automated hepatitis C virus (HCV) core antigen assay as an alternative to real-time PCR for HCV RNA quantification. J Clin Microbiol. 2010. 48:2253–6.
Article24.Ross RS., Viazov S., Salloum S., Hilgard P., Gerken G., Roggendorf M. Analytical performance characteristics and clinical utility of a novel assay for total hepatitis C virus core antigen quantification. J Clin Microbiol. 2010. 48:1161–8.
Article25.Valcavi P., Medici MC., Casula F., Arcangeletti MC., De Conto F., Pinardi F, et al. Evaluation of a total hepatitis C virus (HCV) core antigen assay for the detection of antigenaemia in anti-HCV positive individuals. J Med Virol. 2004. 73:397–403.
Article26.Icardi G., Ansaldi F., Bruzzone BM., Durando P., Lee S., de Luigi C, et al. Novel approach to reduce the hepatitis C virus (HCV) window period: clinical evaluation of a new enzyme-linked immunosorbent assay for HCV core antigen. J Clin Microbiol. 2001. 39:3110–4.
Article27.Buti M., Mendez C., Schaper M., Sauleda S., Valdes A., Rodriguez-Frias F, et al. Hepatitis C virus Core Antigen as a predictor of non-response in genotype 1 chronic hepatitis C patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2004. 40:527–32.28.Maynard M., Pradat P., Berthillon P., Picchio G., Voirin N., Martinot M, et al. Clinical relevance of total HCV core antigen testing for hepatitis C monitoring and for predicting patients' response to therapy. J Viral Hepat. 2003. 10:318–23.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of HCV Core Protein for Detection of HCV Viremia
- Evaluation of Abbott Fourth Generation HIV Antigen and Antibody Assays
- Usefulness of Track-C (Total HCV Core Antigen) Assays in Anti-HCV Positive Patients
- Evaluation of the VIDAS Anti-HCV Assay for Detection of Hepatitis C Virus Infection
- Analytical Performance of New ARCHITECT AFP Assay: Comparison with the Current Assay