Evaluation of the Abbott Cell-Dyn Sapphire Hematology Analyzer
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. cjr0606@yumc.yonsei.ac.kr
- KMID: 1781478
- DOI: http://doi.org/10.3343/kjlm.2007.27.3.162
Abstract
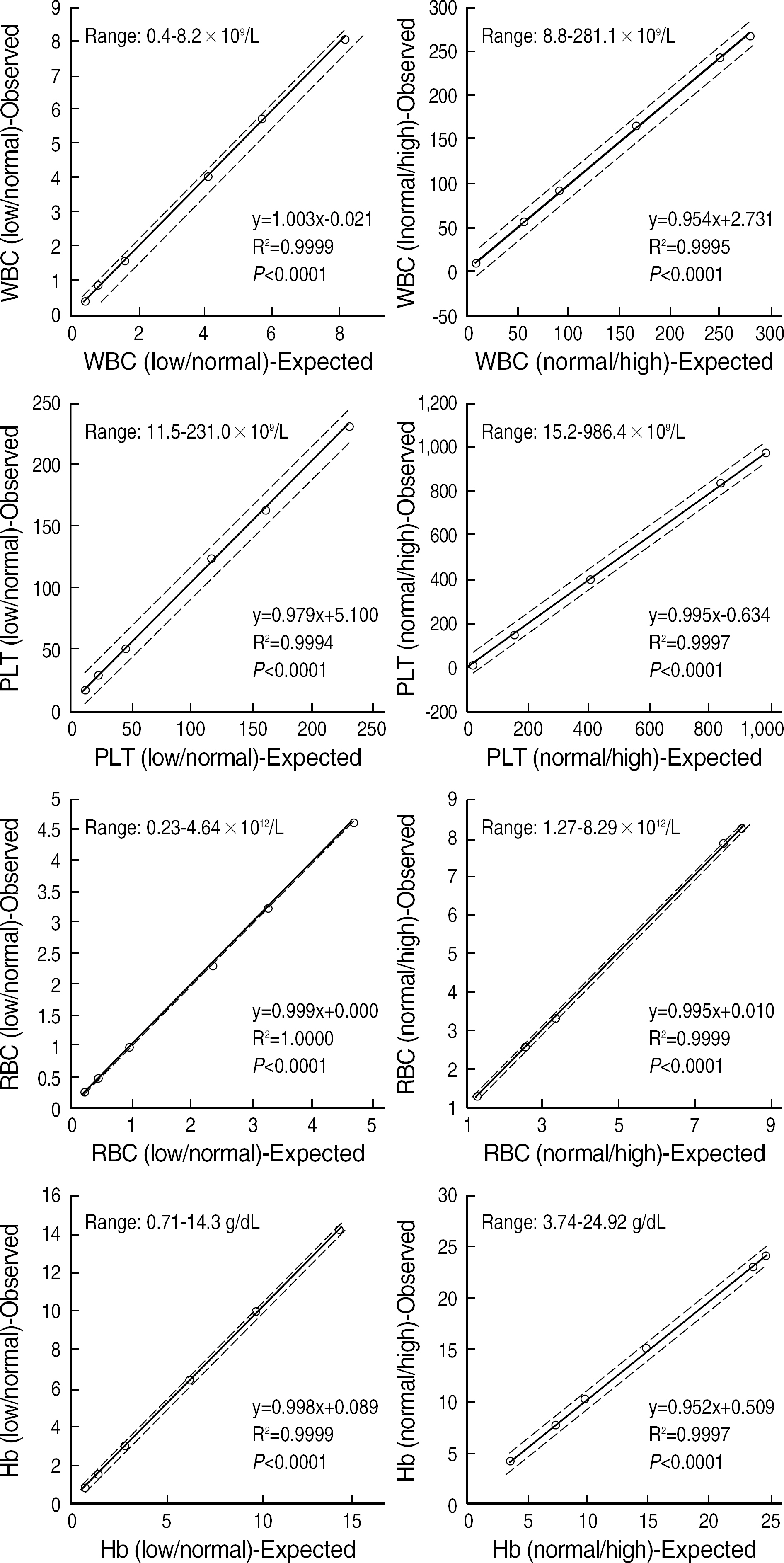
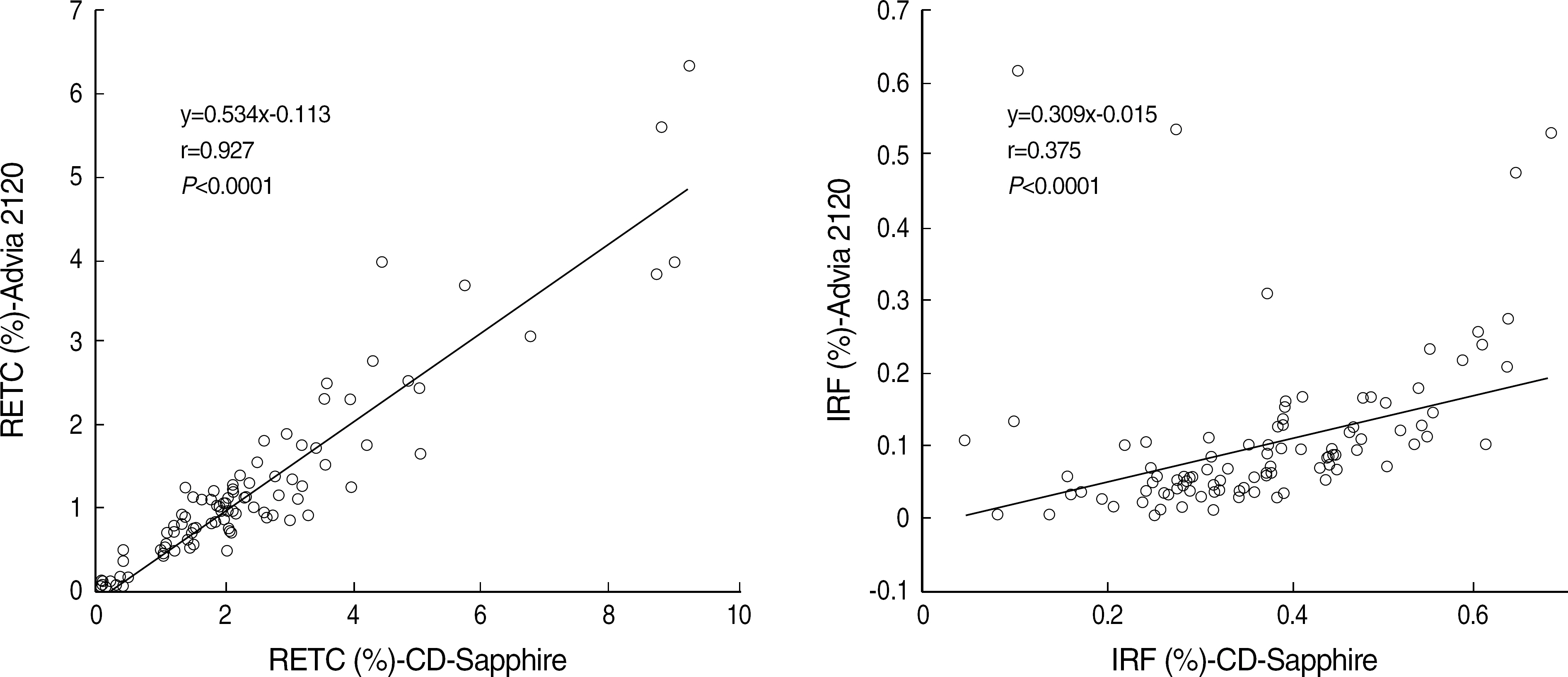
- BACKGROUND: The performance of Cell-Dyn Sapphire (Abbott Diagnostic, USA) was compared to the Bayer Advia 2120 (Bayer Diagnostics, USA), Sysmex XE-2100 (Sysmex Corporation, Japan), and reference microscopy. METHODS: Three hundred samples for routine CBC and WBC differentials were randomly chosen for a comparison analysis. The Cell-Dyn Sapphire system was evaluated according to the linearity, imprecision, inter-instrument correlations, and white blood cell differential. RESULTS: The CBC parameters (WBC, RBC, hemoglobin and platelet) showed a significant linearity with correlation coefficients greater than 0.99 (P<0.0001). Coefficients of variation (CV) for withinrun and differential count of WBC were less than 5% except for Total CV for monocytes, eosinophils, and basophils and within-run CV for low valued eosinophils. The correlation coefficients with manual count were lower in monocytes, eosinophils, and basophils than in neutrophils and lymphocytes. The correlation with other hematology anlayzers was significant exclusive of basophils. CONCLUSIONS: These results demonstrate that the Cell-Dyn Sapphire has a good linearity, an acceptable reproducibility, a minimal carryover, and a comparable performance with the sysmex XE-2100 and Advia 2120.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Letter to the editor: Prognostic significance of preoperative and follow-up neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with non-metastatic clear cell renal cell carcinoma
Beuy Joob, Viroj Wiwaniitkit
Investig Clin Urol. 2019;60(4):331-332. doi: 10.4111/icu.2019.60.4.331.Evaluation of Platelet count by the CELL-DYN Sapphire CD61 Immunoplatelet Method in Patients with Hematologic Diseases Receiving Chemotherapy
Bo-Ram Kim, Jae-Lim Choi, Ji-Eun Kim, Kwang-Sook Woo, Kyeoung-Hee Kim, Jeong-Man Kim, Sung-Hyun Kim, Jin-Yeong Han
Lab Med Online. 2015;5(3):133-136. doi: 10.3343/lmo.2015.5.3.133.Evaluation of the Automated Hematology Analyzer Sysmex XN-2000 and the Accuracy of Differential Leukocyte Counts Using the Low WBC Mode
Ja Young Lee, Sae Am Song, Seung Hwan Oh, Jeong Hwan Shin, Hye Ran Kim, Kyung Ran Jun, Jeong Nyeo Lee
Lab Med Online. 2015;5(4):188-195. doi: 10.3343/lmo.2015.5.4.188.
Reference
-
References
1. Muller R, Mellors I, Johannessen B, Aarsand AK, Kiefer P, Hardy J, et al. European multi-center evaluation of the Abbott Cell-Dyn sapphire hematology analyzer. Lab Hematol. 2006; 12:15–31.2. National Committee for Clinical Laboratory Standards. Reference leukocyte differential count (proportional) and evaluation of instrumental methods. NCCLS Document H20-A.Wayne, Pa: National Committee for Clinical Laboratory Standards;1992. 12:p. 1–55.3. Chae SL, Park JS, Kim DC, Kim SW, Cha YJ. Sysmex SE-9000: Evaluation on the Morphologic Flags and Determination of the Review Criteria. Korean J Clin Pathol. 2000; 20:449–54.4. Grimaldi E, Scopacasa F. Evaluation of the Abbott CELL-DYN 4000 hematology analyzer. Am J Clin Pathol. 2000; 113:497–505.
Article5. Buttarello M, Bulian P, Temporin V, Rizzotti P. Sysmex SE-9000 hematology analyzer: performance evaluation on leukocyte differential counts using an NCCLS H20-A protocol. National Committee for Clinical Laboratory Standards. Am J Clin Pathol. 1997; 108:674–86.6. Aulesa C. Pastor I, Naranjo D, Piqueras J, Galimany R. Validation of the Coulter LH 750 in hospital reference laboratory. Lab Hematol. 2003; 9:15–28.7. Woo HY, Park H. Performance evaluation of the LC-175CRP™ analyzer for determination of complete blood cell count and quantitative C-Reactive Protein. Korean J Lab Med. 2005; 25:1–6.8. Shin S, Kim JE, Yoon JH. Reference Intervals of reticulocyte parameters using ADVIA 120. Korean J Lab Med. 2003; 23:6–11.9. Chang CC, Kass L. Clinical significance of immature reticulocyte fraction determined by automated reticulocyte counting. Am J Clin Pathol. 1997; 108:69–73.
Article10. Johannessen B, Roemer B, Flatmoen L, Just T, Aarsand AK, Scott CS. Implementation of monoclonal antibody fluorescence on the Abbott CELL-DYN Sapphire haematology analyser: evaluation of lymphoid, myeloid and platelet markers. Clin Lab Haematol. 2006; 28:84–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of CELL-DYN Sapphire Hematology Analyzer
- CELL-DYN Sapphire Hematology Analyzer Performance Evaluation on Leukocyte Differential Counts
- Evaluation of Platelet count by the CELL-DYN Sapphire CD61 Immunoplatelet Method in Patients with Hematologic Diseases Receiving Chemotherapy
- Quantitation of T-lymphocyte Subsets Using the CELL-DYN Sapphire Automated Haematology Analyser
- Comparison of the Reagents in Automated Hematology Analyzer Coulter STKS and Cell-Dyn 1300