Ann Lab Med.
2013 May;33(3):174-183. 10.3343/alm.2013.33.3.174.
Flow Cytometric Human Leukocyte Antigen-B27 Typing with Stored Samples for Batch Testing
- Affiliations
-
- 1Department of Clinical Pathology, Kyungpook National University School of Medicine, Daegu, Korea. wondi@knu.ac.kr
- KMID: 1781322
- DOI: http://doi.org/10.3343/alm.2013.33.3.174
Abstract
- BACKGROUND
Flow cytometry (FC) HLA-B27 typing is still used extensively for the diagnosis of spondyloarthropathies. If patient blood samples are stored for a prolonged duration, this testing can be performed in a batch manner, and in-house cellular controls could easily be procured. In this study, we investigated various methods of storing patient blood samples.
METHODS
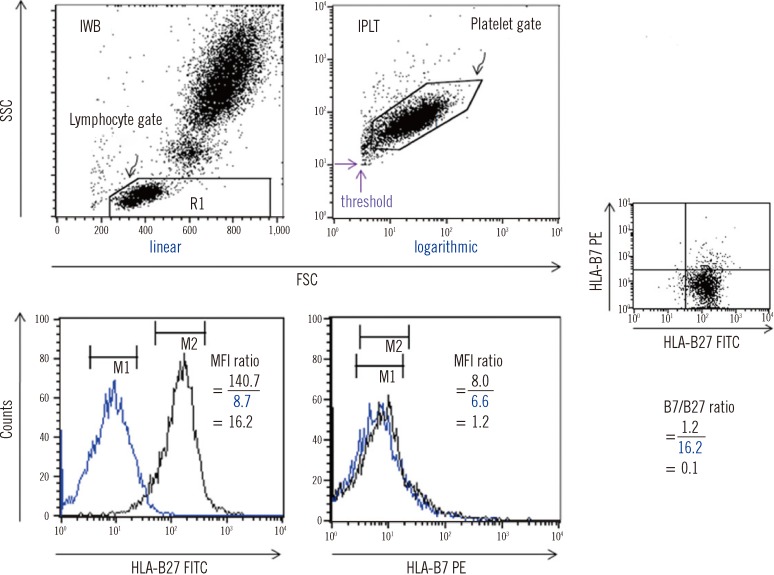
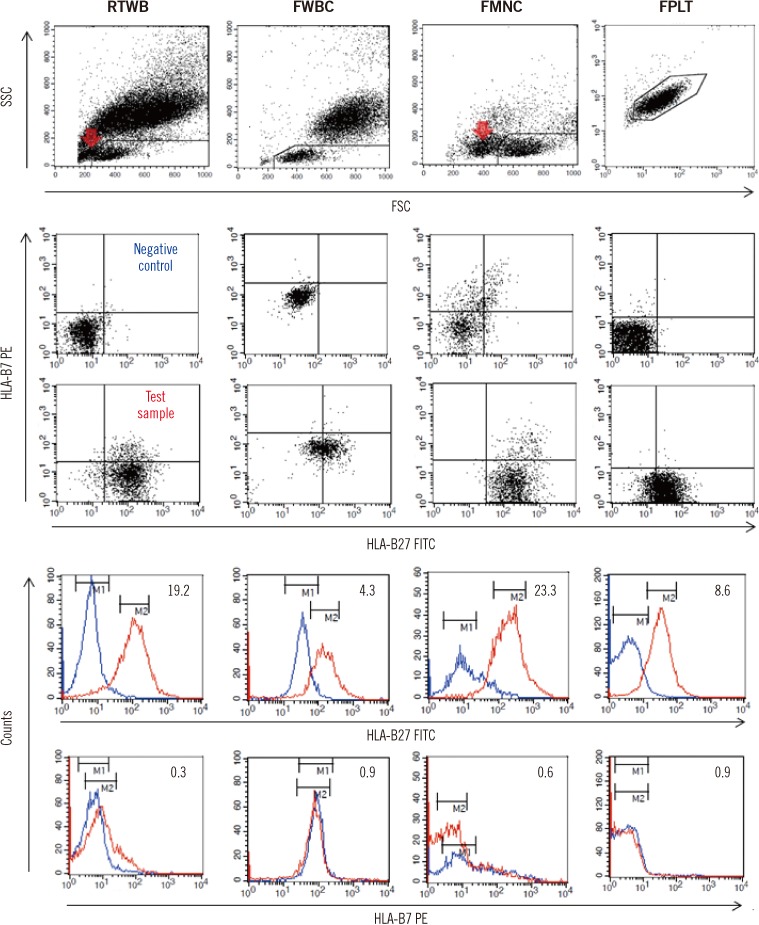
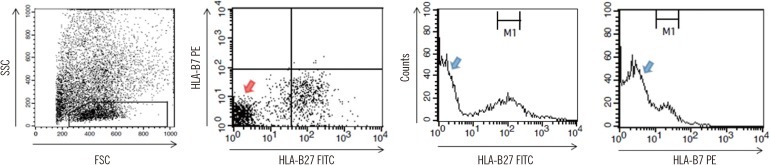
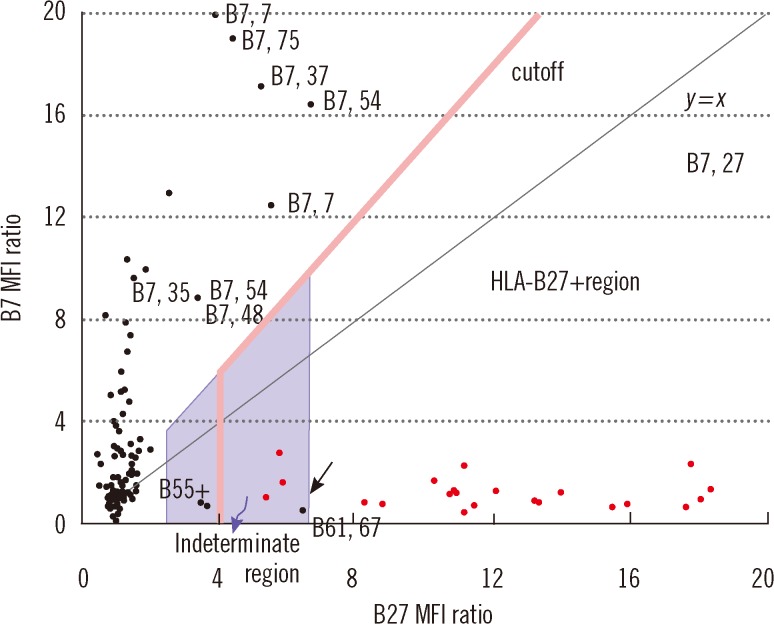
We compared four storage methods: three methods of analyzing lymphocytes (whole blood stored at room temperature, frozen mononuclear cells, and frozen white blood cells [WBCs] after lysing red blood cells [RBCs]), and one method using frozen platelets (FPLT). We used three ratios associated with mean fluorescence intensities (MFI) for HLAB27 assignment: the B27 MFI ratio (sample/control) for HLA-B27 fluorescein-5-isothiocyanate (FITC); the B7 MFI ratio for HLA-B7 phycoerythrin (PE); and the ratio of these two ratios, B7/B27 ratio.
RESULTS
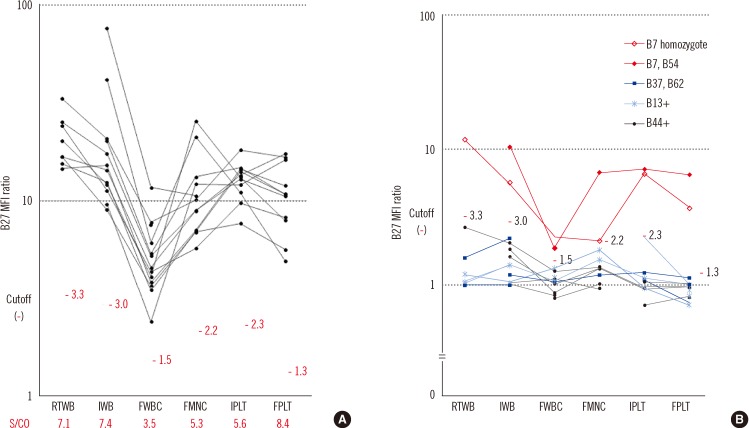
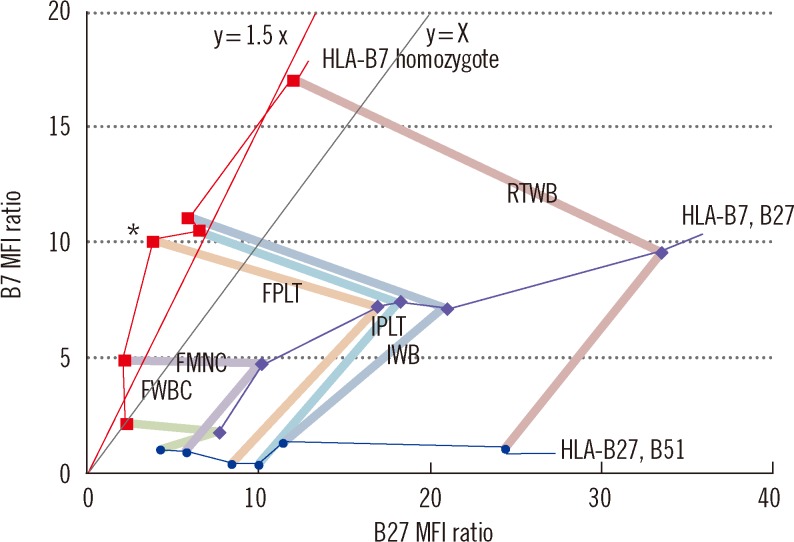
Comparing the B27 MFI ratios of each storage method for the HLA-B27+ samples and the B7/B27 ratios for the HLA-B7+ samples revealed that FPLT was the best of the four methods. FPLT had a sensitivity of 100% and a specificity of 99.3% for HLA-B27 assignment in DNA-typed samples (N=164) when the two criteria, namely, B27 MFI ratio >4.0 and B7/B27 ratio <1.5, were used.
CONCLUSIONS
The FPLT method was found to offer a simple, economical, and accurate method of FC HLA-B27 typing by using stored patient samples. If stored samples are used, this method has the potential to replace the standard FC typing method when used in combination with a complementary DNA-based method.
MeSH Terms
Figure
Reference
-
1. Reveille JD. Major histocompatibility genes and ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2006; 20:601–609. PMID: 16777585.
Article2. Seipp MT, Erali M, Wies RL, Wittwer C. HLA-B27 typing: evaluation of an allele-specific PCR melting assay and two flow cytometric antigen assays. Cytometry B Clin Cytom. 2005; 63:10–15. PMID: 15624199.
Article3. College of American Pathologists. Surveys 2011 B27-A HLA-B27 typing participant summary. 2011. Northfield: College of American Pathologists;p. 1–5. (http://www.cap.org).4. Beckman Coulter. IOTest HLA-B27-FITC/HLA-B7-PE package insert (A07739). Last visit on Jan 2013. http://www.beckmancoulter.com/wsrportal/page/itemDetails?itemNumber=A07739#2/10//0/25/1/0/asc/2/A07739///0/1//0/.5. Levering WH, Wind H, Granger V, Sintnicolaas K, Hooijkaas H, Reilly JT, et al. Long-term stabilized blood samples as controls for flow cytometric HLA-B27 screening: a feasibility study. Cytometry B Clin Cytom. 2008; 74:169–181. PMID: 18200592.
Article6. European Federation for Immunogenetics. Standards for Histocompatibility Testing. 2005. 4. Version 5.4.7. American Society for Histocompatibility and Immunogenetics. Revised Standards for Histocompatibility Testing. 2005. 12.8. Levering WH, Sintnicolaas K, Wind H, Hooijkaas H, Gratama JW. Flow cytometric screening for the HLA-B27 antigen on peripheral blood lymphocytes. Curr Protoc Cytom. 2005; 8. Chapter 6: Unit6.22.9. Coates E, Darke C. Routine HLA-B27 typing by flow cytometry: differentiation of the products of HLA-B*2702, B*2705 and B*2708. Eur J Immunogenet. 1998; 25:29–37. PMID: 9587742.
Article10. Coates E, Rees TJ, Darke C. An evaluation of Com-B27, a fluorescein-conjugated mouse anti-HLA-B27 reagent. Eur J Immunogenet. 2003; 30:271–274. PMID: 12919288.
Article11. Rodey GE, Revels K, Fuller TC. Epitope specificity of HLA class I alloantibodies: II. Stability of cross-reactive group antibody patterns over extended time periods. Transplantation. 1997; 63:885–893. PMID: 9089230.12. McFarland JG. Roback JD, Combs MR, Grossman BJ, Hillyer CD, editors. Platelet and granulocyte antigens. Technical manual. 2008. 16th ed. Bethesda: AABB;p. 525–546.13. Helmerhorst FM, ten Berge ML, van der Plas-van Dalen CM, Engelfriet CP, von dem Borne AE. Platelet freezing for serological purposes with and without a cryopreservative. Vox Sang. 1984; 46:318–322. PMID: 6375132.
Article14. National Institute for Biological Standards and Control. Platelet immunofluorescence test protocol. Last visit on Oct 2012. http://www.nibsc.ac.uk/science/diagnostics/transfusion__transplantation/platelets/resources/pift_protocol.aspx.15. Moroff G, Seetharaman S, Kurtz JW, Greco NJ, Mullen MD, Lane TA, et al. Retention of cellular properties of PBPCs following liquid storage and cryopreservation. Transfusion. 2004; 44:245–252. PMID: 14962316.
Article16. Maecker HT, Moon J, Bhatia S, Ghanekar SA, Maino VC, Payne JK, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005; 6:17. PMID: 16026627.
Article17. Cho EH, Lee SG, Seok JH, Park BY, Lee EH. Evaluation of two commercial HLA-B27 real-time PCR kits. Korean J Lab Med. 2009; 29:589–593. PMID: 20046093.
Article18. McKenna RM, Takemoto SK. Improving HLA matching for kidney transplantation by use of CREGs. Lancet. 2000; 355:1842–1843. PMID: 10866435.
Article19. Porretti L, Marangoni F, Rebulla P, Sirchia G. Frozen platelet plates for platelet antibody detection and cross-match. Vox Sang. 1994; 67:52–57. PMID: 7975453.
Article20. Darke C, Coates E. One-tube HLA-B27/B2708 typing by flow cytometry using two "Anti-HLA-B27" monoclonal antibody reagents. Cytometry B Clin Cytom. 2010; 78:21–30. PMID: 19693889.
Article21. Lee SH, Choi IA, Lee YA, Park EK, Kim YH, Kim KS, et al. Human leukocyte antigen-B*2705 is the predominant subtype in the Korean population with ankylosing spondylitis, unlike in other Asians. Rheumatol Int. 2008; 29:43–46. PMID: 18493767.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of HLA-B27 typing methods -PCR-SSP, microlymphocytotoxicity, and flow cytometry
- Evaluation of a Korean HLA-B27 typing tray
- Comparison of the Flowcytometric HLA-B27 Determination Methods
- Annual Report of Korean Association of External Quality Assessment Service on Histocompatibility Testing (2018)
- The Comparison of Duration to Maintain Cell Viability for HLA-B27 Test According to Anticoagulants