Yonsei Med J.
2014 Jan;55(1):70-77. 10.3349/ymj.2014.55.1.70.
Feasibility and Outcomes of Hypofractionated Simultaneous Integrated Boost-Intensity Modulated Radiotherapy for Malignant Gliomas: A Preliminary Report
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Korea. jjhmd@yuhs.ac
- 2Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1779888
- DOI: http://doi.org/10.3349/ymj.2014.55.1.70
Abstract
- PURPOSE
The aim of this study was to assess the feasibility and efficacy of hypofractionated simultaneous integrated boost-intensity modulated radiotherapy (SIB-IMRT) using three-layered planning target volumes (PTV) for malignant gliomas.
MATERIALS AND METHODS
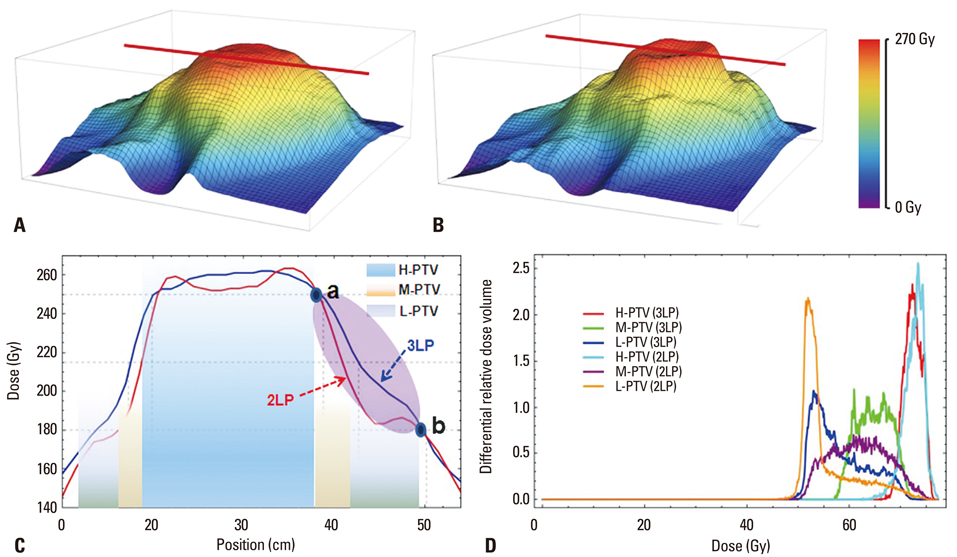
We conducted a retrospective analysis of 12 patients (WHO grade IV-10; III-2) postoperatively treated with SIB-IMRT with concurrent temozolomide. Three-layered PTVs were contoured based on gadolinium-enhanced magnetic resonance imaging as follows; high risk PTV (H-PTV) as the area of surgical bed including residual gross tumor with a 0.5 cm margin; low risk PTV (L-PTV) as the area surrounding the high risk PTV with 1.5 cm margin; moderate risk PTV (M-PTV) as a line at one-third the distance from high risk PTV to low risk PTV. Total dose to high risk PTV was 70 Gy in 8 and 62.5 Gy in 4 patients.
RESULTS
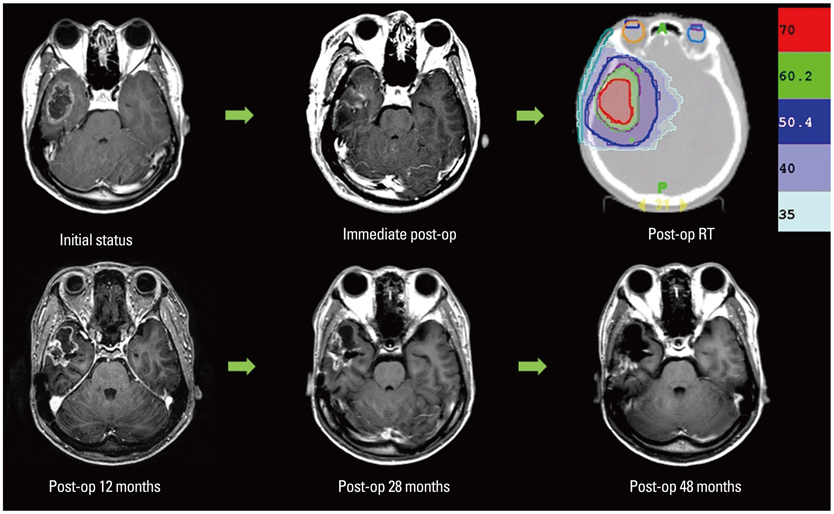
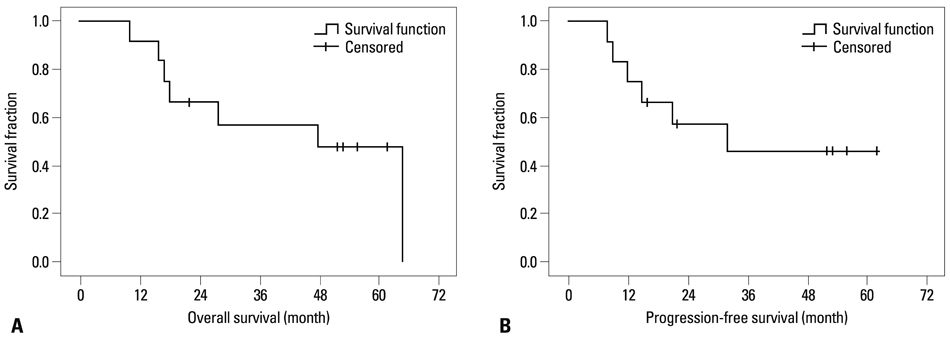
The median follow-up time was 52 months in surviving patients. The 2- and 5-year overall survival (OS) rates were 66.6% and 47.6%, respectively. The 2- and 5-year progression-free survival (PFS) rates were 57.1% and 45.7%, respectively. The median OS and PFS were 48 and 31 months, respectively. Six patients (50%) progressed: in-field only in one, out-field or disseminated in 4, and both in one patient. All patients completed planned treatments without a toxicity-related gap. Asymptomatic radiation necrosis was observed in 4 patients at post-radiotherapy 9-31 months.
CONCLUSION
An escalated dose of hypofractionated SIB-IMRT using three-layered PTVs can be safely performed in patients with malignant glioma, and might contribute to better tumor control and survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee SW, Fraass BA, Marsh LH, Herbort K, Gebarski SS, Martel MK, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999; 43:79–88.
Article2. Selker RG, Shapiro WR, Burger P, Blackwood MS, Arena VC, Gilder JC, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002; 51:343–355.
Article3. Prados MD, Wara WM, Sneed PK, McDermott M, Chang SM, Rabbitt J, et al. Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001; 49:71–77.
Article4. Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004; 60:853–860.
Article5. MacDonald SM, Ahmad S, Kachris S, Vogds BJ, DeRouen M, Gittleman AE, et al. Intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for the treatment of high grade glioma: a dosimetric comparison. J Appl Clin Med Phys. 2007; 8:47–60.
Article6. Chan MF, Schupak K, Burman C, Chui CS, Ling CC. Comparison of intensity-modulated radiotherapy with three-dimensional conformal radiation therapy planning for glioblastoma multiforme. Med Dosim. 2003; 28:261–265.
Article7. Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2012.8. Apuzzo MLJ. AANS Publications Committee. Malignant cerebral glioma. Park Ridge, Ill: American Association of Neurological Surgeons;1990.9. Phillips TL, Hoppe R, Roach M, Leibel SA. Leibel and Phillips textbook of radiation oncology. 3rd ed. Philadelphia: Elsevier/Saunders;2010.10. Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002; 20:1375–1382.
Article11. Haas-Kogan DA, Yount G, Haas M, Levi D, Kogan SS, Hu L, et al. p53-dependent G1 arrest and p53-independent apoptosis influence the radiobiologic response of glioblastoma. Int J Radiat Oncol Biol Phys. 1996; 36:95–103.
Article12. Chan JL, Lee SW, Fraass BA, Normolle DP, Greenberg HS, Junck LR, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002; 20:1635–1642.
Article13. Narayana A, Yamada J, Berry S, Shah P, Hunt M, Gutin PH, et al. Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys. 2006; 64:892–897.
Article14. Floyd NS, Woo SY, Teh BS, Prado C, Mai WY, Trask T, et al. Hypofractionated intensity-modulated radiotherapy for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004; 58:721–726.
Article15. Iuchi T, Hatano K, Narita Y, Kodama T, Yamaki T, Osato K. Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys. 2006; 64:1317–1324.
Article16. Stupp R, Mason WP, van den, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.
Article17. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466.
Article18. Cao JQ, Fisher BJ, Bauman GS, Megyesi JF, Watling CJ, Macdonald DR. Hypofractionated radiotherapy with or without concurrent temozolomide in elderly patients with glioblastoma multiforme: a review of ten-year single institutional experience. J Neurooncol. 2012; 107:395–405.
Article19. Yang X, Darling JL, McMillan TJ, Peacock JH, Steel GG. Radiosensitivity, recovery and dose-rate effect in three human glioma cell lines. Radiother Oncol. 1990; 19:49–56.
Article20. Shibamoto Y, Nishimura Y, Tsutsui K, Sasai K, Takahashi M, Abe M. Comparison of accelerated hyperfractionated radiotherapy and conventional radiotherapy for supratentorial malignant glioma. Jpn J Clin Oncol. 1997; 27:31–36.
Article21. Romanelli P, Conti A, Pontoriero A, Ricciardi GK, Tomasello F, De Renzis C, et al. Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg Focus. 2009; 27:E8.
Article22. Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. 2007; 85:339–343.
Article23. Burnet NG, Jena R, Jefferies SJ, Stenning SP, Kirkby NF. Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin Oncol (R Coll Radiol). 2006; 18:93–103.
Article24. Kim YS, Kim SH, Cho J, Kim JW, Chang JH, Kim DS, et al. MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys. 2012; 84:661–667.
Article25. Suzuki M, Nakamatsu K, Kanamori S, Okumra M, Uchiyama T, Akai F, et al. Feasibility study of the simultaneous integrated boost (SIB) method for malignant gliomas using intensity-modulated radiotherapy (IMRT). Jpn J Clin Oncol. 2003; 33:271–277.
Article26. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006; 1:2315–2319.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term oncological outcomes of hypofractionated versus conventional fractionated whole breast irradiation with simultaneous integrated boost in early-stage breast cancer
- Effectiveness and feasibility of concurrent chemoradiotherapy using simultaneous integrated boost-intensity modulated radiotherapy with and without induction chemotherapy for locally advanced pancreatic cancer
- Hypofractionated radiotherapy for early glottic cancer: a retrospective interim analysis of a single institution
- A randomized prospective study comparing acute toxicity, compliance and objective response rate between simultaneous integrated boost and sequential intensity-modulated radiotherapy for locally advanced head and neck cancer
- Simultaneous integrated boost intensity-modulated radiotherapy versus 3-dimensional conformal radiotherapy in preoperative concurrent chemoradiotherapy for locally advanced rectal cancer