Yonsei Med J.
2007 Apr;48(2):289-294. 10.3349/ymj.2007.48.2.289.
Black Cohosh and St. John's Wort (GYNO-Plus(R)) for Climacteric Symptoms
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea. kh8730@yumc.yonsei. ac.kr
- 2Department of Obstetrics and Gynecology, Ewha Woman's University College of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Konyang University College of Medicine, Daejeon, Korea.
- 4Department of Pathology, Ewha Woman's University College of Medicine, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Chonnam National University Medical School, Kwangju, Korea.
- KMID: 1779533
- DOI: http://doi.org/10.3349/ymj.2007.48.2.289
Abstract
- PURPOSE
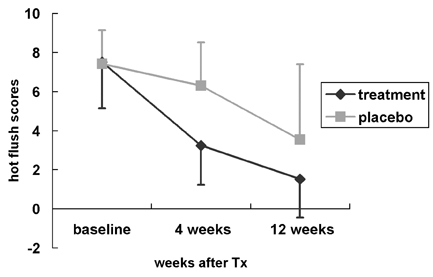
This study was conducted to investigate the efficacy of black cohosh (Cimicifuga racemosa) and St. John's wort (Hypericum perforatum) in women with climacteric symptoms, and to assess their effects on vaginal atrophy, hormone levels, and lipid profiles. MATERIALS AND METHODS: In this double-blind randomized, placebo-controlled, multicenter study, 89 peri- or postmenopausal women experiencing climacteric symptoms were treated with St. John's wort and black cohosh extract (Gynoplus
Keyword
MeSH Terms
Figure
Reference
-
1. Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994. 29:319–336.2. Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001. 65:125–134.3. Herrington DM, Klein KP. Cardiovascular trials of estrogen replacement therapy. Ann N Y Acad Sci. 2001. 949:153–162.4. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998. 280:605–613.5. Liehr JG. Genotoxicity of the steroidal oestrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Hum Reprod Update. 2001. 7:273–281.6. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health initiative randomized controlled trial. JAMA. 2002. 288:321–333.7. Wade C, Kronenberg F, Kelly A, Murphy PA. Hormone-modulating herbs: implications for women's health. J Am Med Womens Assoc. 1999. 54:181–183.8. Dantas SM. Menopausal symptoms and alternative medicine. Prim Care Update Ob Gyns. 1999. 6:212–220.9. Burdette JE, Liu J, Chen SN, Fabricant DS, Piersen CE, Barker EL, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003. 51:5661–5670.10. Lieberman S. A review of the effectiveness of Cimicifuga racemosa (black cohosh) for the symptoms of menopause. J Womens Health. 1998. 7:525–529.11. Stoll W. Phytopharmaceutical influences of atrophic vaginal epithelium: double-blind study on Cimifuga vs. an estrogen preparation. Therapeutikon. 1987. 1:23–31.12. Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, Kinne D, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001. 19:2739–2745.13. Wuttke W, Seidlova-Wuttke D, Gorkow C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: effects on menopause symptoms and bone markers. Maturitas. 2003. 44:Suppl 1. S67–S77.14. Uebelhack R, Blohmer JU, Graubaum HJ, Busch R, Gruenwald J, Wernecke KD. Black cohosh and St. John's wort for climacteric complaints: a randomized trial. Obstet Gynecol. 2006. 107:247–255.15. Kupperman KI, Blatt MHG, Wiesbader H, Filler W. Comparative clinical evaluation of estrogenic preparations by the menopausal and amenorrheal indices. J Clin Endocrinol. 1953. 13:688–703.16. Lehmann-Willenbrock E, Riedel HH. Clinical and endocrinologic studies of the treatment of ovarian insufficiency manifestations following hysterectomy with intact adnexa. Zentralbl Gynakol. 1988. 110:611–618.17. Pockaj BA, Gallagher JG, Loprinzi CL, Stella PJ, Barton DL, Sloan JA, et al. Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG trial N01CC. J Clin Oncol. 2006. 24:2836–2841.18. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005. 23:117–125.19. Jarry H, Metten M, Spengler B, Christoffel V, Wuttke W. In vitro effects of the Cimicifuga racemosa extract BNO 1055. Maturitas. 2003. 44:Suppl 1. S31–S38.20. Gardiner SA, Morrison MF, Mozley PD, Mozley LH, Brensinger C, Bilker W, et al. Pilot study on the effect of estrogen replacement therapy on brain dopamine transporter availability in healthy, postmenopausal women. Am J Geriatr Psychiatry. 2004. 12:621–630.21. Wuttke W, Gorkow C, Seidlova-Wuttke D. Effects of black cohosh (Cimicifuga racemosa) on bone turnover, vaginal mucosa, and various blood parameters in postmenopausal women: a double-blind, placebo-controlled, and conjugated estrogens-controlled study. Menopause. 2006. 13:185–196.22. Raus K, Brucker C, Gorkow C, Wuttke W. First-time proof of endometrial safety of the special black cohosh extract (Actaea or Cimicifuga racemosa extract) CR BNO 1055. Menopause. 2006. 13:678–691.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The efficacy and safety of the combined preparation of Black Cohosh and St. John's wort in menopausal women
- The Effect of black cohosh with St. John's wort (Feramin-Q(R)) on climacteric symptoms: multicenter randomized double-blind placebo-controlled trial
- Effect of Black Cohosh on Genital Atrophy and Its Adverse Effect in Postmenopausal Women
- The short-term effect of black cohosh on vaginal atrophy and safety in postmenopausal women
- Relationship between Type D Personality, Climacteric Symptoms, Climacteric Symptoms Management, and Well-being among Middle-aged Women