J Korean Med Sci.
2006 Jun;21(3):572-576. 10.3346/jkms.2006.21.3.572.
A Case of Non-Functioning Huge Adrenocortical Carcinoma Extending Into Inferior Vena Cava and Right Atrium
- Affiliations
-
- 1Department of Cardiovascular Medicine, Chonnam National University Hospital, Chonnam National University Research Institute of Medical Sciences, Gwangju, Korea. jcpark@chonnam.ac.kr
- 2Department of Hemato-oncology, Chonnam National University Hospital, Chonnam National University Research Institute of Medical Sciences, Gwangju, Korea.
- KMID: 1778448
- DOI: http://doi.org/10.3346/jkms.2006.21.3.572
Abstract
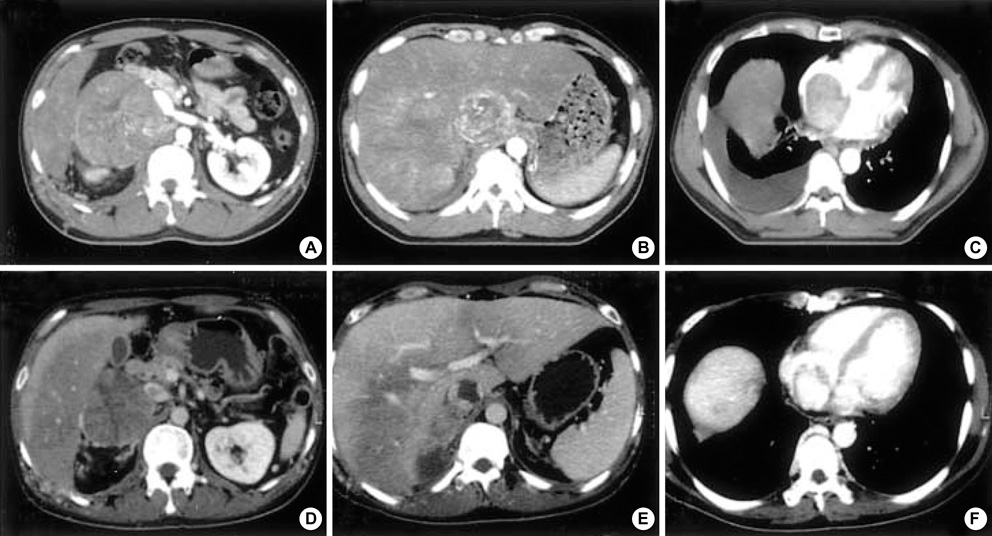
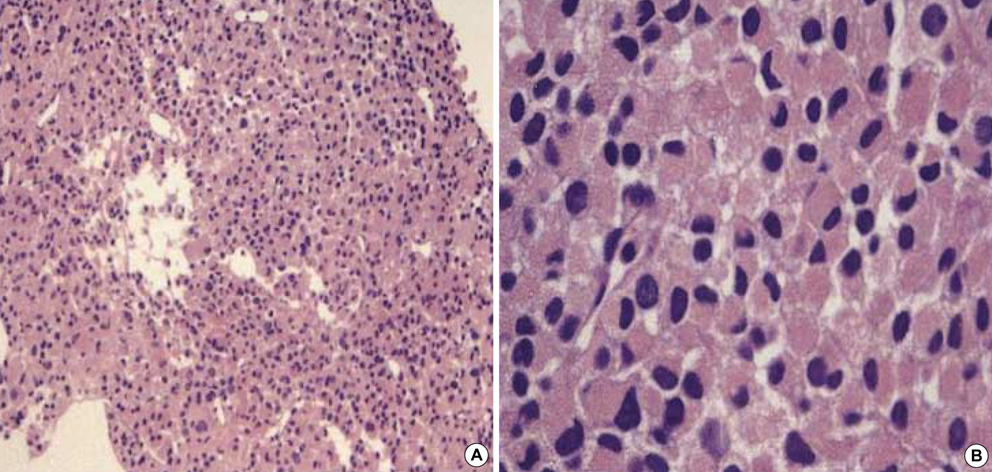
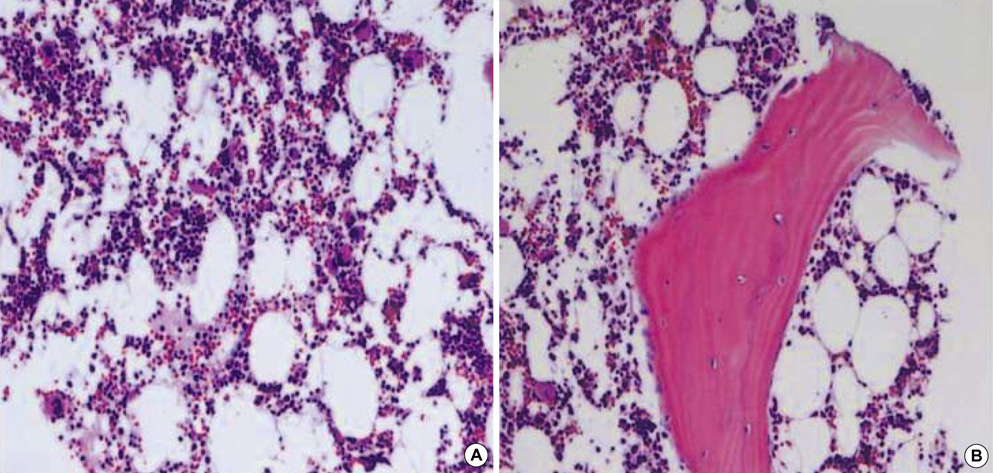
- Primary adrenocortical carcinoma (ACC) is a rare tumor and its usual sites of metastasis are the lung (71%), lymph node (68%), liver (42%), and bone (26%). However, intracaval invasion extending into the right atrium is very rare and spontaneous regression of tumor burden in adrenal carcinoma is also rare. We report a case of ACC with direct invasion of the inferior vena cava and right atrium. A 34-yr-old male patient presented with progressive dyspnea, weight loss, and poor oral intake over 3 months. Non-functioning ACC with direct invasion of the inferior vena cava and right atrium was confirmed by imaging, pathologic, and hormonal study. Chemo-radiotherapy was attempted. However, tumor burden was not changed, but rather toxic hepatitis and thrombocytopenia were developed. His subjective symptoms and general conditions were improved after 1 month of conservative management and the patient was discharged. During clinical follow-up, this tumor showed spontaneous regression.
MeSH Terms
Figure
Reference
-
1. Reynen K, Kockeritz U, Strasser RH. Metastases to the heart. Ann Oncol. 2004. 15:375–381.
Article2. McClennan BL. Staging and follow-up of renal and adrenal carcinoma. Cancer. 1991. 67:1199–1208.3. Norton JA, Le HN. DeVita VT, editor. Adrenal tumors. Cancer. 2001. 6th ed. Philadelphia: Lippincott Williams & Wilkins;1770–1785.4. Stojadinovic A, Ghossein RA, Hoos A, Nissan A, Marshall D, Dudas M, Cordon-Cardo C, Jaques DP, Brennan MF. Adrenocortical carcinoma: Clinical, morphologic, and molecular characterization. J Clin Oncol. 2002. 20:941–950.
Article5. Schramek P, Dunser E, Bhargabha A, Hurby W, Umek H. Adrenal cortical carcinoma: preoperative demonstration of right atrial extension by sonography and computerized tomography. J Urol. 1985. 133:260–262.
Article6. Okazumi S, Asano T, Ryu M, Nagashima T, Odaka M, Isono K, Nishizawa T. Surgical resection of adrenal carcinoma extending into vena cava, right atrium and ventricle: Case report and review of the literature. Nippon Geka Gekkai Zasshi. 1987. 88:231–238.7. Shahian DM, Nieh PT, Libertino JA. Resection of atriocaval adrenal carcinoma using hypothermic circulatory arrest. Ann Thorac Surg. 1989. 48:421–422.
Article8. Galli R, Parlapiano M, Pace Napoleone C, Pierangeli A. Neoplastic caval and intracardiac thrombosis secondary to reno-adrenal tumors. One-stage surgical treatment in deep hypothermia and circulatory arrest. Minerva Urol Nefrol. 1994. 46:105–111.9. Bilge A, Pierard LA, Kulbertus HE. Isolated cardiac metastasis of adrenal carcinoma: Transesophageal echocardiographic features. Am Heart J. 1996. 132:1066–1068.
Article10. Yamana D, Yanagi T, Nanbu I, Tanaka K, Hirabayashi S, Tohyama J, Mizutani H, Ohba S, Katoh N, Ono Y. Intracaval invasion of left adrenal cortical carcinoma extending into the right atrium. Radiat Med. 1997. 15:327–330.11. Hedican SP, Marshall FF. Adrenocortical carcinoma with intracaval extension. J Urol. 1997. 158:2056–2061.
Article12. Salinas Sanchez AS, Segura Martin M, Lorenzo Romero JG, Hernandez-Millan I, Albertos Salvador J, Virseda Rodriuez JA. Adrenal cortex carcinoma with right atrium involvement. Surgery with cardiopulmonary bypass. Actas Urol Esp. 2000. 24:590–593.13. Chesson JP, Theodorescu D. Adrenal tumor with caval extension. Scan J Urol Nephrol. 2002. 36:71–73.14. Rosen B, Rozenman Y, Harpaz D. Extension of adrenocortical carcinoma into the right atrium-echocardiographic diagnosis: A case report. Cardiovasc Ultrasound. 2003. 1:5.
Article15. Kaiser HE, Bodey B Jr, Siegel SE, Groger AM, Bodey B. Spontaneous neoplastic regression: the significance of apoptosis. In Vivo. 2000. 14:773–788.16. Cui Z, Willingham MC, Hicks AM, Alexander-Miller MA, Howard TD, Hawkins GA, Miller MS, Weir HM, Du W, DeLong CJ. Spontaneous regression of advanced cancer: Identification of a unique genetically determined, age-dependent trait in mice. Proc Natl Acad Sci USA. 2003. 100:6682–6687.
Article17. Chang KC, Chan KL, Lam CW. Spontaneous regression of renal cell metastases. Hong Kong Med J. 1999. 5:72–75.18. Seo HJ, Jung JH, Song YT. Spontaneous regression of liver metastasis in stage IV-S neuroblastoma after adrenalectomy: one case report. J Korean Assoc Pediatr Surg. 2001. 7:68–72.19. Saracco S, Abramowsky C, Taylor S, Silverman RA, Berman BW. Spontaneously regressing adrenocortical carcinoma in a newborn. A case report with DNA ploidy analysis. Cancer. 1988. 62:507–511.
Article20. Gohji K, Kizaki T, Fujii A. Spontaneous regression of pulmonary metastasis from nonfunctioning adrenocortical carcinoma after removal of the primary lesion: a case report. J Urol. 1995. 154:1854–1855.
Article21. Holgersen LO, Subramanian S, Kirpekar M, Mootabar H, Marcus JR. Spontaneous resolution of antenatally diagnosed adrenal masses. J Pediatr Surg. 1996. 31:153–155.
Article22. Kasat LS, Borwankar SS, Naregal A, Jain M. Complete spontaneous regression of a functioning adrenocortical carcinoma in an infant. Pediatr Surg Int. 2001. 17:230–231.
Article23. Allolio B, Hahner S, Weismann D, Fassnacht M. Management of adrenocortical carcinoma. Clin Endoclinol (Oxf). 2004. 60:273–287.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Removal of Renal Cell Carcinoma Extending Into the Inferior Vena Cava and Right Atrium using Hypothermia and Circulatory Arrest
- Hepatocellular Carcinoma Extending to the Inferior Vena Cava and Right Atrium-A Case Report of 4 Years Survival after Repeated Transcatheter Arterial Chemoembolization Therapy -
- Small Renal Cell Carcinoma Associated with Inferior Vena Cava Thrombus
- Anesthetic Management for Liver Segmentectomy and Thrombectomy of Inferior Vena Cava and Right Atrium without Cardiopulmonary Bypass : A case report
- Renal Cell Carcinoma with Absence of Inferior Vena Cava