J Korean Med Sci.
2006 Jun;21(3):460-468. 10.3346/jkms.2006.21.3.460.
Heterogeneity of Invasive Ductal Carcinoma: Proposal for a Hypothetical Classification
- Affiliations
-
- 1Department of General Surgery, Miz Medi Hospital, Seoul, Korea. drjo514@yahoo.co.kr
- 2Department of Anatomic Pathology, Samsung Cheil Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1778429
- DOI: http://doi.org/10.3346/jkms.2006.21.3.460
Abstract
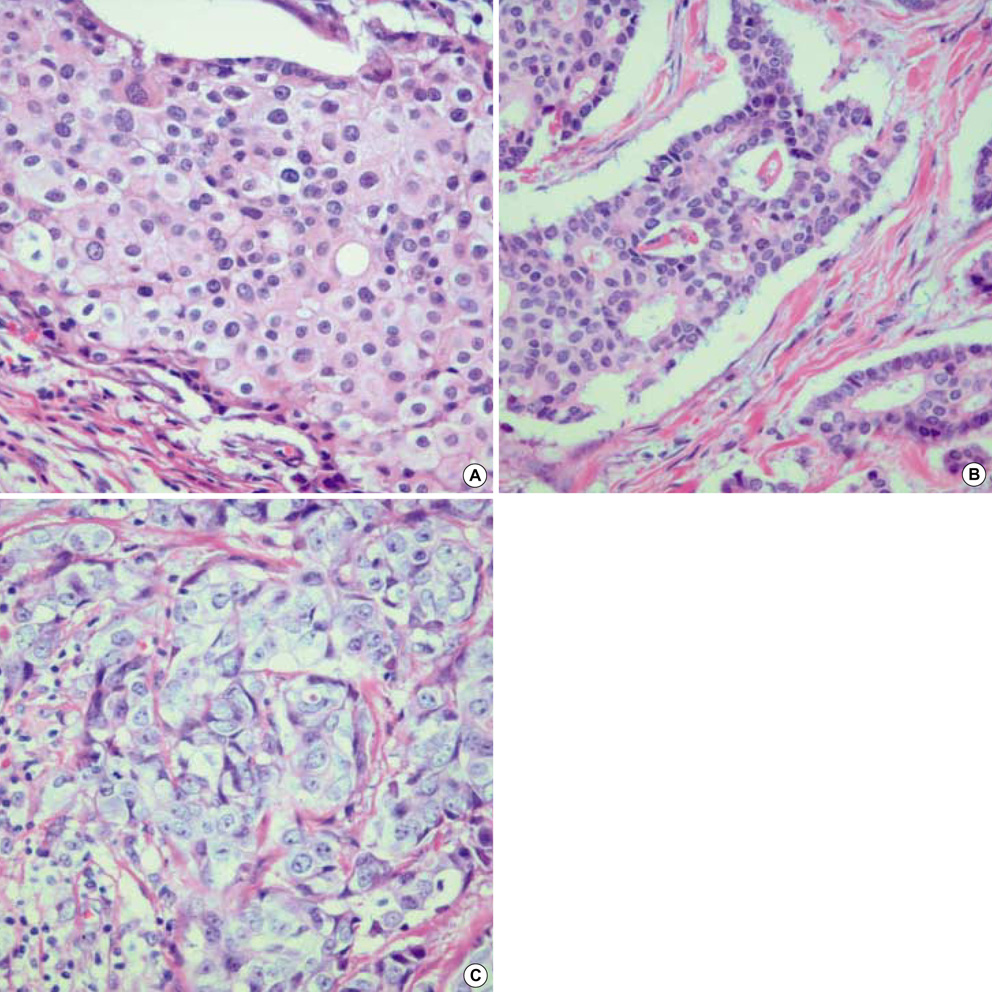
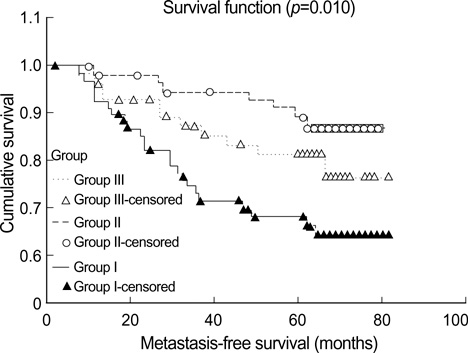
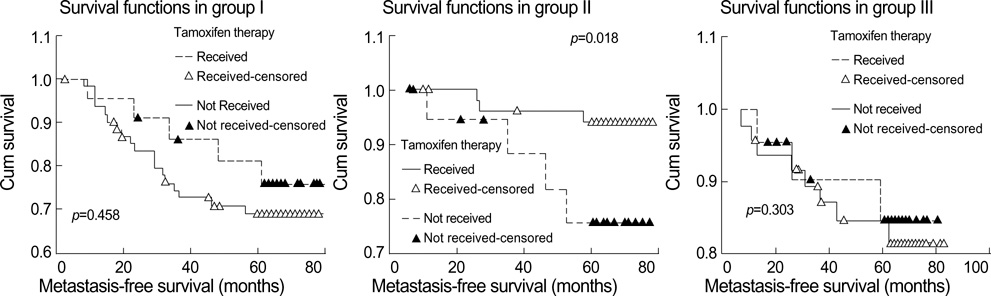
- To investigate what heterogeneity exists in breast cancer, 228 consecutive patients with operable invasive duetal carcinoma (IDC), not otherwise specified, were categorized on the basis of the horizontal progression model of carcinogenesis. Using the reversed Black's nuclear grade (RBNG) in the IDC component and the association of ductal carcinoma in situ (DCIS), the patients were classified into pure IDC (IDC de novo or ab initio) as Group I, non-high grade (RBNG 1 and 2) IDC with DCIS as Group II, and high grade (RBNG 3) IDC with DCIS as Group III. The Groups classified in the present study appeared as a prognostic factor independent of known prognostic and predictive factors in multivariate test. Group I had the worst prognosis among the three groups and was the most non-responsive to tamoxifen. After performing stratifying analyses by group, it was found that metastasis-free survival was statistically associated with the status of hormone receptors estrogen receptor and progesterone receptor and tamoxifen therapy only in Group II. In addition, the status of c-erbB-2 expression had prognostic significance only within the Group III. Our results may be used to frame an alternative hypothetical model for breast cancer evolution and will lead us to reconsider the tailoring of the comprehensive therapeutic modality used at the present time.
Keyword
MeSH Terms
-
Treatment Outcome
Time Factors
Receptors, Progesterone/metabolism
Receptors, Estrogen/metabolism
Prognosis
Neoplasm Metastasis
Middle Aged
Humans
Female
Disease-Free Survival
*Classification
Cell Nucleus/metabolism
Carcinoma, Ductal, Breast/*classification/*diagnosis/pathology
Breast Neoplasms/*classification/*diagnosis/pathology
Aged
Adult
Figure
Reference
-
1. Sant M, Allemani C, Berrino F, Coleman MP, Aareleid T, Chaplain G, Coebergh JW, Colonna M, Crosignani P, Danzon A, Federico M, Gafa L, Grosclaude P, Hedelin G, Mace-Lesech J, Garcia CM, Moller H, Paci E, Raverdy N, Tretarre B, Williams EM. European Concerted Action on Survival and Care of Cancer Patients (EUROCARE) Working Group. European Concerted Action on Survival and Care of Cancer Patients (EUROCARE) Working Group. Breast carcinoma survival in Europe and the United States. Cancer. 2004. 100:715–722.2. Bloom HJ, Richardson WW, Harries EJ. Natural history of untreated breast cancer (1805-1933). Comparison of untreated and treated cases according to histological grade of malignancy. Br Med J. 1962. 5299:213–221.3. Johnstone PA, Norton MS, Riffenburgh RH. Survival of patients with untreated breast cancer. J Surg Oncol. 2000. 73:273–277.
Article4. Fox MS. On the diagnosis and treatment of breast cancer. JAMA. 1979. 241:489–494.
Article5. Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988. 319:525–532.
Article6. O'Connell P, Pekkel V, Fuqua S, Osborne CK, Allred DC. Molecular genetic studies of early breast cancer evolution. Breast Cancer Res Treat. 1994. 32:5–12.7. Buerger H, Otterbach F, Simon R, Poremba C, Diallo R, Decker T, Riethdorf L, Brinkschmidt C, Dockhorn-Dworniczak B, Boecker W. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol. 1999. 187:396–402.8. Mirza AN, Mirza NQ, Vlastos G, Singletary SE. Prognostic factors in node-negative breast cancer: a review of studies with sample size more than 200 and follow-up more than 5 years. Ann Surg. 2002. 235:10–26.9. Heimann R, Hellman S. Clinical progression of breast cancer malignant behavior: what to expect and when to expect it. J Clin Oncol. 2000. 18:591–599.
Article10. Clark GM. Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Prognostic factors and Predictive Factors. Diseases of the breast. 2000. Philadelphia: Lippincott Williams & Wilkins;489–514.11. Fisher ER, Redmond C, Fisher B. Histologic grading of breast cancer. Pathol Annu. 1980. 15:239–251.12. Bose S, Mohammed M, Shintaku P, Rao PN. Her-2/neu gene amplification in low to moderately expressing breast cancers: possible role of chromosome 17/Her-2/neu polysomy. Breast J. 2001. 7:337–344.
Article13. Bocker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, Burger H, Wai D, Ina Diallo R, Brandt B, Herbst H, Schmidt A, Lerch MM, Buchwallow IB. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab Invest. 2002. 82:737–746.
Article14. Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF, Nicholson RI, Ellis IO. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004. 203:661–671.
Article15. O'Connell P, Pekkel V, Fuqua SA, Osborne CK, Clark GM, Allred DC. Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst. 1998. 90:697–703.16. Farabegoli F, Champeme MH, Bieche I, Santini D, Ceccarelli C, Derenzini M, Lidereau R. Genetic pathways in the evolution of breast ductal carcinoma in situ. J Pathol. 2002. 196:280–286.17. Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003. 100:8418–8423.18. Silverberg SG, Chitale AR. Assessment of significance of proportions of intraductal and infiltrating tumor growth in ductal carcinoma of the breast. Cancer. 1973. 32:830–837.
Article19. Lampejo OT, Barnes DM, Smith P, Millis RR. Evaluation of infiltrating ductal carcinomas with a DCIS component: correlation of the histologic type of the in situ component with grade of the infiltrating component. Semin Diagn Pathol. 1994. 11:215–222.20. Elston CW, Gresham GA, Rao GS, Zebro T, Haybittle JL, Houghton J, Kearney G. The cancer research campaign (King's/Cambridge trial for early breast cancer: clinico-pathological aspects). Br J Cancer. 1982. 45:655–669.
Article21. Heimann R, Hellman S. Individual characterisation of the metastatic capacity of human breast carcinoma. Eur J Cancer. 2000. 36:1631–1639.
Article22. Engel J, Eckel R, Kerr J, Schmidt M, Furstenberger G, Richter R, Sauer H, Senn HJ, Holzel D. The process of metastasisation for breast cancer. Eur J Cancer. 2003. 39:1794–1806.
Article23. Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957. 105:97–102.24. Leong AS, Sormunen RT, Vinyuvat S, Hamdani RW, Suthipintawong C. Biologic markers in ductal carcinoma in situ and concurrent infiltrating carcinoma. A comparison of eight contemporary grading systems. Am J Clin Pathol. 2001. 115:709–718.25. Douglas-Jones AG, Gupta SK, Attanoos RL, Morgan JM, Mansel RE. A critical appraisal of six modern classifications of ductal carcinoma in situ of the breast (DCIS): correlation with grade of associated invasive carcinoma. Histopathology. 1996. 29:397–409.
Article26. Moreno A, Lloveras B, Figueras A, Escobedo A, Ramon JM, Sierra A, Fabra A. Ductal carcinoma in situ of the breast: correlation between histologic classifications and biologic markers. Mol Pathol. 1997. 10:1088–1092.27. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953. 6:963–968.
Article28. Van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, Mross F, Dieterich H, Seitz R, Ross D, Botstein D, Brown P. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002. 161:1991–1996.
Article29. Man YG, Tai L, Barner R, Vang R, Saenger JS, Shekitka KM, Bratthauer GL, Wheeler DT, Liang CY, Vinh TN, Strauss BL. Cell clusters overlying focally disrupted mammary myoepithelial cell layers and adjacent cells within the same duct display different immunohistochemical and genetic features: implications for tumor progression and invasion. Breast Cancer Res. 2003. 5:R231–R241.
Article30. Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, Bryant J, Dimitrov NV, Abramson N, Atkins JN, Shibata H, Deschenes L, Margolese RG. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997. 89:1673–1682.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ductal Carcinoma In Situ of the Breast: Comparison of Histologic Classifications and Correlation with Histologic Grade of Coexisting Invasive Ductal Carcinoma
- Clinical Features of Invasive Ductal Carcinoma According to Histopathologic Classification
- Invasive Lobular Carcinoma of the Breast Associated with Mixed Lobular and Ductal Carcinoma In Situ: A Case Report
- An Unusual Presentation of Extensive Ductal Carcinoma in Situ Accompanying Invasive Ductal Carcinoma on MRI: A Case Report
- Synchronous Encapsulated Papillary Carcinoma and Invasive Ductal Carcinoma Arising from Intraductal Papilloma in the Same Breast: A Case Report