Korean J Gastroenterol.
2012 Oct;60(4):229-241. 10.4166/kjg.2012.60.4.229.
Significance of Preoperative Tissue Levels of Vascular-endothelial Cadherin, Liver-intestine Cadherin and Vascular Endothelial Growth Factor in Gastric Cancer
- Affiliations
-
- 1Center for Cancer Prevention and Detection, National Cancer Center, Goyang, Korea.
- 2Department of Internal Medicine, Ewha Medical Research Institute, Ewha Womans University School of Medicine, Seoul, Korea. shimkn@ewha.ac.kr
- 3Department of Surgery, Ewha Medical Research Institute, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 1775807
- DOI: http://doi.org/10.4166/kjg.2012.60.4.229
Abstract
- BACKGROUND/AIMS
The aims of this study were to examine the expressions of endothelium specific VE-cadherin, intestine specific LI-cadherin, and vascular endothelial growth factor (VEGF), and to determine their relationships with the clinicopathological parameters of gastric cancer.
METHODS
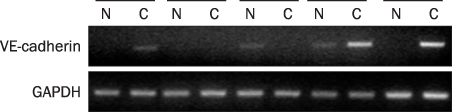
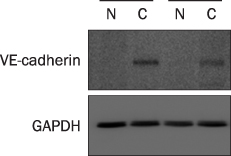
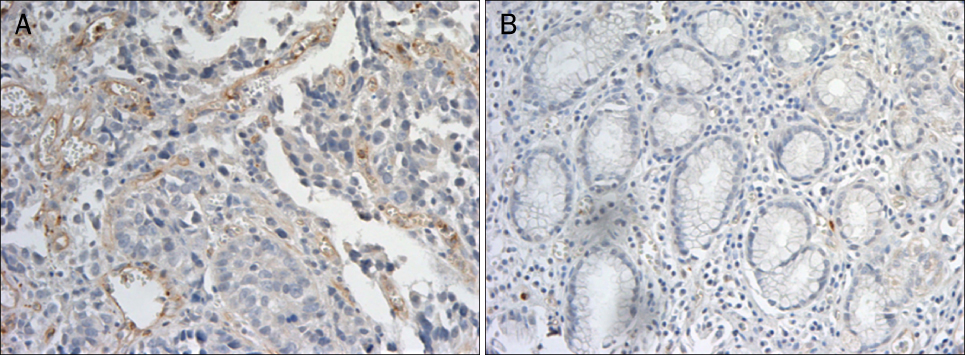
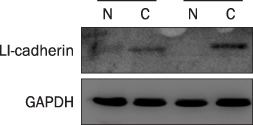
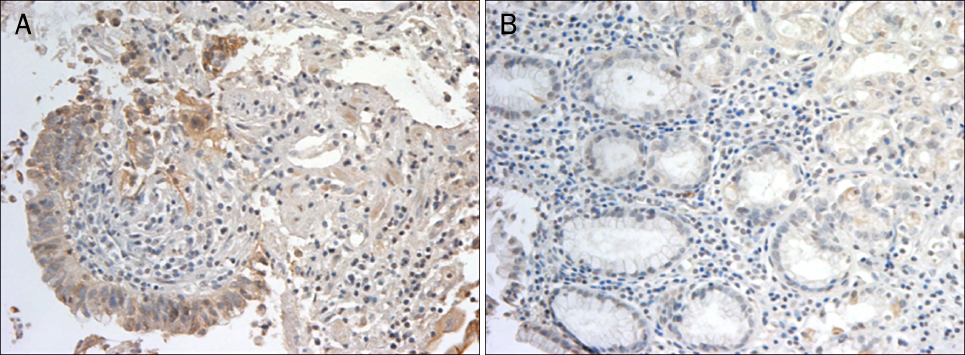
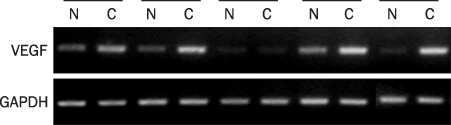
A total 47 patients with gastric cancer who underwent surgery were enrolled. Endoscopic biopsies were obtained from the cancer and normal mucosa, respectively. Using semiquantitative RT-PCR, the mRNA expression levels of VE-cadherin, LI-cadherin and VEGF were measured by tumor/normal (T/N) ratios. The protein expressions of VE-cadherin, LI-cadherin and VEGF were examined by Western blot and immunohistochemical stain in surgically resected tissues. The clinicopathological variables were reviewed and analyzed, retrospectively.
RESULTS
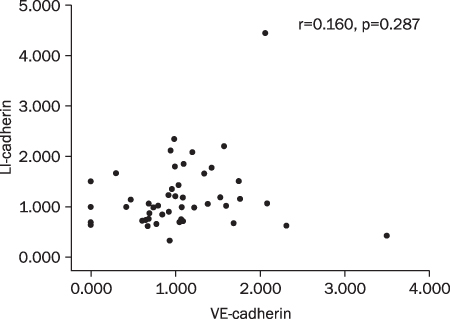
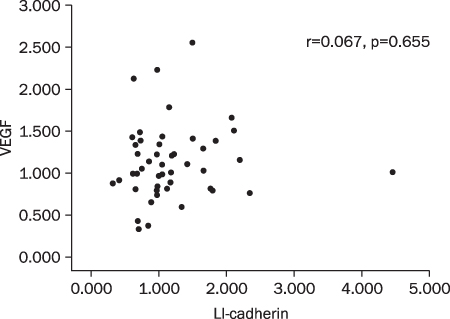
Twenty two cases (46.8%) of VE-cadherin, 25 cases (53.2%) of LI-cadherin and 27 cases (51.1%) of VEGF mRNA expressions were overexpressed in gastric cancer compared to normal tissue. There was a tendency for T/N ratio of VE-cadherin mRNA to correlate with the lymphatic invasion (p=0.07) and the lymph node metastasis (p=0.099) in advanced gastric cancer. The T/N ratio of LI-cadherin mRNA showed significant association with distant metastasis (p=0.031) and lymphatic invasion especially in advanced gastric cancer (p=0.023). There was a tendency for the T/N ratio of VEGF mRNA to correlate with the distant metastasis (p=0.073) in advanced gastric cancer.
CONCLUSIONS
As increased mRNA expression of LI-cadherin was associated with distant metastasis and lymphatic invasion especially in the biopsy specimen of advanced gastric cancer before surgery, it may provide useful preoperative information on tumor aggressiveness.
MeSH Terms
Figure
Reference
-
1. Ministry for Health Welfare and Family Affairs. Yearbook of health and welfare statistics (1993-2007). 2009. Seoul: Ministry of Health and Welfare (Korea).2. Park SS, Kang SH, Park JM, et al. Expression of liver-intestine cadherin and its correlation with lymph node metastasis in gastric cancer: can it predict N stage preoperatively? Ann Surg Oncol. 2007. 14:94–99.3. Grötzinger C, Kneifel J, Patschan D, et al. LI-cadherin: a marker of gastric metaplasia and neoplasia. Gut. 2001. 49:73–81.4. Konturek PC, Konturek SJ, Brzozowski T. Gastric cancer and Helicobacter pylori infection. J Physiol Pharmacol. 2006. 57:Suppl 3. 51–65.5. Cavallaro U, Liebner S, Dejana E. Endothelial cadherins and tumor angiogenesis. Exp Cell Res. 2006. 312:659–667.6. Blaschuk OW, Devemy E. Cadherins as novel targets for anti-cancer therapy. Eur J Pharmacol. 2009. 625:195–198.7. Kato K, Takada T, Fukusato T. Expression of vascular endothelial-cadherin in human hepatocellular carcinoma tissues. Hepatol Res. 2007. 37:444–453.8. Dong WG, Yu QF, Xu Y, Fan LF. Li-cadherin is inversely correlated with galectin-3 expression in gastric cancer. Dig Dis Sci. 2008. 53:1811–1817.9. Ko S, Chu KM, Luk JM, et al. Overexpression of LI-cadherin in gastric cancer is associated with lymph node metastasis. Biochem Biophys Res Commun. 2004. 319:562–568.10. Carmeliet P, Collen D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann N Y Acad Sci. 2000. 902:249–262.11. Lampugnani MG, Dejana E. Adherens junctions in endothelial cells regulate vessel maintenance and angiogenesis. Thromb Res. 2007. 120:Suppl 2. S1–S6.12. George SJ, Dwivedi A. MMPs, cadherins, and cell proliferation. Trends Cardiovasc Med. 2004. 14:100–105.13. Brantjes H, Barker N, van Es J, Clevers H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol Chem. 2002. 383:255–261.14. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991. 251:1451–1455.15. Gessner R, Tauber R. Intestinal cell adhesion molecules. Liver-intestine cadherin. Ann N Y Acad Sci. 2000. 915:136–143.16. Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000. 14:1169–1180.17. Loges S, Clausen H, Reichelt U, et al. Determination of micro-vessel density by quantitative real-time PCR in esophageal cancer: correlation with histologic methods, angiogenic growth factor expression, and lymph node metastasis. Clin Cancer Res. 2007. 13:76–80.18. Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003. 15:509–514.19. Labelle M, Schnittler HJ, Aust DE, et al. Vascular endothelial cadherin promotes breast cancer progression via transforming growth factor beta signaling. Cancer Res. 2008. 68:1388–1397.20. Sulkowska M, Famulski W, Wincewicz A, et al. Levels of VE-cadherin increase independently of VEGF in preoperative sera of patients with colorectal cancer. Tumori. 2006. 92:67–71.21. Ko S, Chu KM, Luk JM, et al. CDX2 co-localizes with liver-intestine cadherin in intestinal metaplasia and adenocarcinoma of the stomach. J Pathol. 2005. 205:615–622.22. Leung WK, Sung JJ. Review article: intestinal metaplasia and gastric carcinogenesis. Aliment Pharmacol Ther. 2002. 16:1209–1216.23. Filipe MI, Potet F, Bogomoletz WV, et al. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut. 1985. 26:1319–1326.24. Osborn M, Mazzoleni G, Santini D, Marrano D, Martinelli G, Weber K. Villin, intestinal brush border hydrolases and keratin polypeptides in intestinal metaplasia and gastric cancer; an immunohistologic study emphasizing the different degrees of intestinal and gastric differentiation in signet ring cell carcinomas. Virchows Arch A Pathol Anat Histopathol. 1988. 413:303–312.25. Ito R, Oue N, Yoshida K, et al. Clinicopathological significant and prognostic influence of cadherin-17 expression in gastric cancer. Virchows Arch. 2005. 447:717–722.26. Takamura M, Sakamoto M, Ino Y, et al. Expression of liver-intestine cadherin and its possible interaction with galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci. 2003. 94:425–430.27. Dong W, Yu Q, Xu Y. Altered expression of a Li-cadherin in gastric cancer and intestinal metaplasia. Dig Dis Sci. 2007. 52:536–542.28. Su MC, Yuan RH, Lin CY, Jeng YM. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod Pathol. 2008. 21:1379–1386.29. Takamura M, Ichida T, Matsuda Y, et al. Reduced expression of liver-intestine cadherin is associated with progression and lymph node metastasis of human colorectal carcinoma. Cancer Lett. 2004. 212:253–259.30. Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006. 8:1223–1234.31. Bazas VM, Lukyanova NY, Demash DV, Galakhin KO, Myasoedov DV. Relation between cell-to-cell adhesion and angiogenesis and clinico-morphological prognostic factors in patients with gastric cancer. Exp Oncol. 2008. 30:235–239.32. Aoyagi K, Kouhuji K, Yano S, et al. VEGF significance in peritoneal recurrence from gastric cancer. Gastric Cancer. 2005. 8:155–163.33. Dagnaes-Hansen F, Rasmussen LM, Tilton R, Denner L, Flyvbjerg A. A murine vascular endothelial growth factor antibody inhibits in vivo growth of human Caki-I renal adenocarcinoma. Anticancer Res. 2003. 23:1625–1630.34. Rabascio C, Muratori E, Mancuso P, et al. Assessing tumor angio-genesis: increased circulating VE-cadherin RNA in patients with cancer indicates viability of circulating endothelial cells. Cancer Res. 2004. 64:4373–4377.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Correlation between the Expression of E-cadherin, VEGF-C, VEGF-D and the Real Extent of Lymph Node Metastases using Cytokeratin 18 in Early Gastric Cancer
- Expression of E-cadherin, Matrix Metalloproteinase, and Vascular Endothelial Growth Factor in Squamous Cell Carcinoma and Adenocarcinoma of the Lung
- Expression of Vascular Endothelial Growth Factor : Clinical Implications in Cervical Neoplasia
- An Immunohistochemical Study of Vascular Endothelial Growth Factor as a Predictor of Progression in Bladder Cancer
- Serum vascular endothelial growth factor as a marker of asthma exacerbation