J Korean Med Sci.
2014 Apr;29(4):587-592. 10.3346/jkms.2014.29.4.587.
Analysis on Bilateral Hindlimb Mapping in Motor Cortex of the Rat by an Intracortical Microstimulation Method
- Affiliations
-
- 1Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. srjeon@amc.seoul.kr
- 2Department of Neurosurgery, Wooridul Spine Hospital, Seoul, Korea.
- 3Department of Neurosurgery, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 4Department of Computer Science and Engineering, University of Notre Dame, Notre Dame, IN, USA.
- KMID: 1774467
- DOI: http://doi.org/10.3346/jkms.2014.29.4.587
Abstract
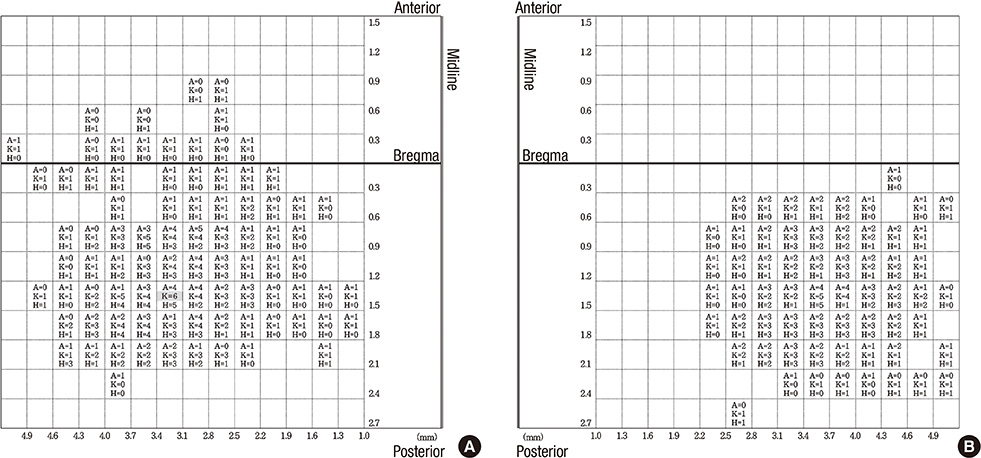
- Intracortical microstimulation (ICMS) is a technique that was developed to derive movement representation of the motor cortex. Although rats are now commonly used in motor mapping studies, the precise characteristics of rat motor map, including symmetry and consistency across animals, and the possibility of repeated stimulation have not yet been established. We performed bilateral hindlimb mapping of motor cortex in six Sprague-Dawley rats using ICMS. ICMS was applied to the left and the right cerebral hemisphere at 0.3 mm intervals vertically and horizontally from the bregma, and any movement of the hindlimbs was noted. The majority (80%+/-11%) of responses were not restricted to a single joint, which occurred simultaneously at two or three hindlimb joints. The size and shape of hindlimb motor cortex was variable among rats, but existed on the convex side of the cerebral hemisphere in all rats. The results did not show symmetry according to specific joints in each rats. Conclusively, the hindlimb representation in the rat motor cortex was conveniently mapped using ICMS, but the characteristics and inter-individual variability suggest that precise individual mapping is needed to clarify motor distribution in rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Asanuma H, Rosén I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972; 14:243–256.2. Asanuma H, Ward JE. Patterns of contraction of distal forelimb muscles produced by intracortical stimulation in cats. Brain Res. 1971; 27:97–109.3. Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998; 80:3321–3325.4. Kolb B, Tees RC. The cerebral cortex of the rat. Cambridge: MIT Press;1990.5. Young NA, Vuong J, Flynn C, Teskey GC. Optimal parameters for microstimulation derived forelimb movement thresholds and motor maps in rats and mice. J Neurosci Methods. 2011; 196:60–69.6. Gharbawie OA, Williams PT, Kolb B, Whishaw IQ. Transient middle cerebral artery occlusion disrupts the forelimb movement representations of rat motor cortex. Eur J Neurosci. 2008; 28:951–963.7. Young NA, Vuong J, Ozen LJ, Flynn C, Teskey GC. Motor map expansion in the pilocarpine model of temporal lobe epilepsy is dependent on seizure severity and rat strain. Exp Neurol. 2009; 217:421–428.8. Telfeian AE, Connors BW. Widely integrative properties of layer 5 pyramidal cells support a role for processing of extralaminar synaptic inputs in rat neocortex. Neurosci Lett. 2003; 343:121–124.9. Fonoff ET, Pereira JF Jr, Camargo LV, Dale CS, Pagano RL, Ballester G, Teixeira MJ. Functional mapping of the motor cortex of the rat using transdural electrical stimulation. Behav Brain Res. 2009; 202:138–141.10. Hosp JA, Molina-Luna K, Hertler B, Atiemo CO, Stett A, Luft AR. Thin-film epidural microelectrode arrays for somatosensory and motor cortex mapping in rat. J Neurosci Methods. 2008; 172:255–262.11. Molina-Luna K, Buitrago MM, Hertler B, Schubring M, Haiss F, Nisch W, Schulz JB, Luft AR. Cortical stimulation mapping using epidurally implanted thin-film microelectrode arrays. J Neurosci Methods. 2007; 161:118–125.12. Xie N, Yang Q, Chappell TD, Li CX, Waters RS. Prenatal alcohol exposure reduces the size of the forelimb representation in motor cortex in rat: an intracortical microstimulation (ICMS) mapping study. Alcohol. 2010; 44:185–194.13. Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, Margrie TW. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. J Comp Neurol. 2004; 479:360–373.14. Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982; 212:76–88.15. Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011; 21:865–876.16. Frost SB, Iliakova M, Dunham C, Barbay S, Arnold P, Nudo RJ. Reliability in the location of hindlimb motor representations in Fischer-344 rats: laboratory investigation. J Neurosurg Spine. 2013; 19:248–255.17. VandenBerg PM, Hogg TM, Kleim JA, Whishaw IQ. Long-Evans rats have a larger cortical topographic representation of movement than Fischer-344 rats: a microstimulation study of motor cortex in naïve and skilled reaching-trained rats. Brain Res Bull. 2002; 59:197–203.18. Gu X, Staines WA, Fortier PA. Quantitative analyses of neurons projecting to primary motor cortex zones controlling limb movements in the rat. Brain Res. 1999; 835:175–187.19. Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986; 396:77–96.20. Gioanni Y, Lamarche M. A reappraisal of rat motor cortex organization by intracortical microstimulation. Brain Res. 1985; 344:49–61.21. Weiss DS, Keller A. Specific patterns of intrinsic connections between representation zones in the rat motor cortex. Cereb Cortex. 1994; 4:205–214.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The After-effect of Sub-threshold 10 Hz Repetitive Transcranial Magnetic Stimulation on Motor Cortical Excitability
- The Motor Cortex Mapping Using Transcranial Magnetic Stimulation in Stroke Patients
- Changes in Activity of Human Motor Cortex Caused by Hyperbaric Air Therapy
- Machine Learning-assisted Quantitative Mapping of Intracortical Axonal Plasticity Following a Focal Cortical Stroke in Rodents
- The Optimal Condition for Activation of Motor Cortex by Peripheral Electrical Stimulation in Rat