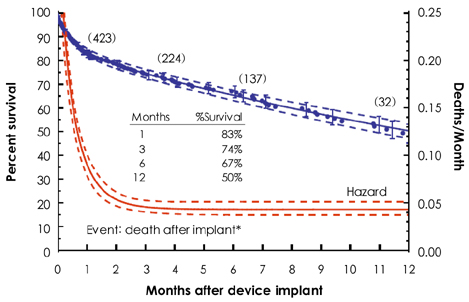
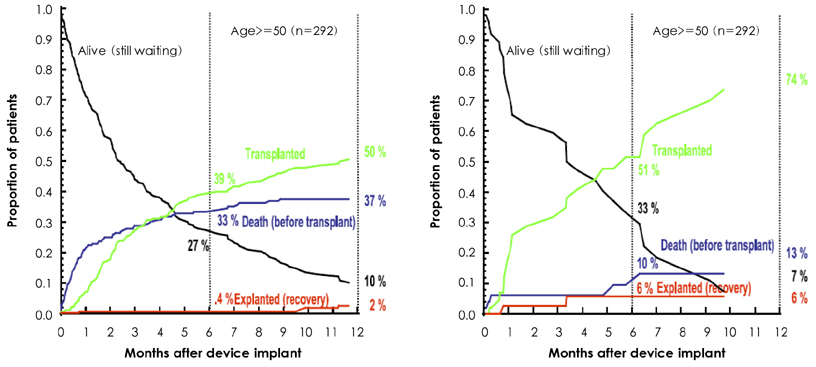
Korean Circ J.
2009 Jan;39(1):1-10. 10.4070/kcj.2009.39.1.1.
Current Mechanical Circulatory Support Devices for End Stage Heart Failure
- Affiliations
-
- 1Department of Cardiovascular Surgery, Yonsei Cardiovascular Hospital, Yonsei University Health System, Seoul, Korea. yhpark@yuhs.ac
- KMID: 1769506
- DOI: http://doi.org/10.4070/kcj.2009.39.1.1
Abstract
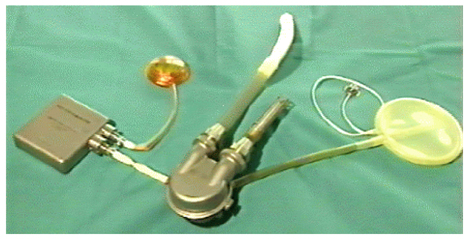
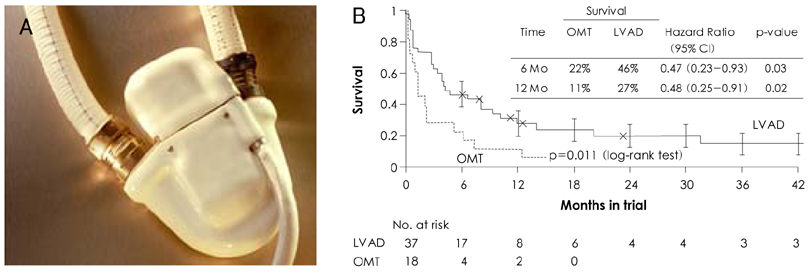
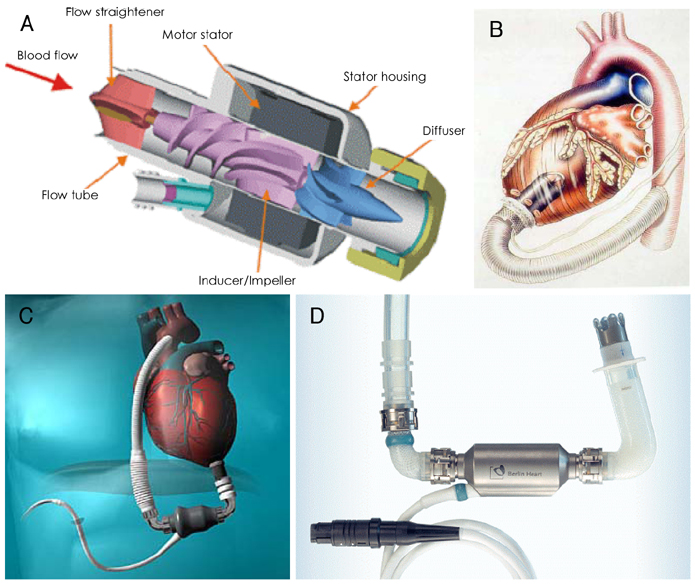
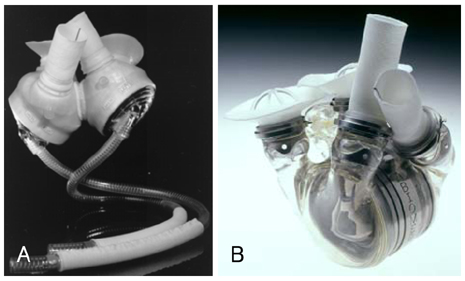
- Mechanical circulatory support is necessary when heart failure becomes refractory to medical support. It is typically instituted when organ dysfunction occurs as a result of hypoperfusion. Enthusiasm has recently developed for the role of mechanical circulatory support in the ever-growing population of heart failure patients. Indeed, efforts in developing this technology have allowed for the relatively recent development of a variety of complete circulatory support devices. The use of left ventricular assist devices (LVADs) in patients with advanced heart failure results in a clinically meaningful survival benefit and an improved quality of life, and LVADs could be an acceptable alternative therapy for selected patients who are not candidates for cardiac transplantation.
MeSH Terms
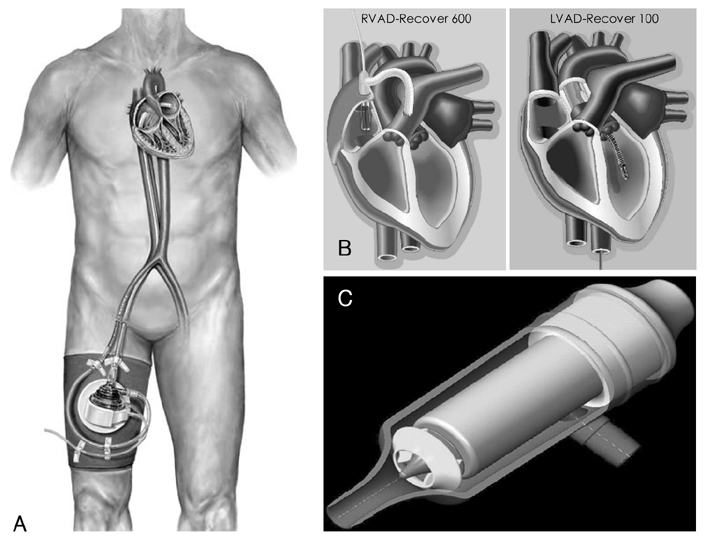
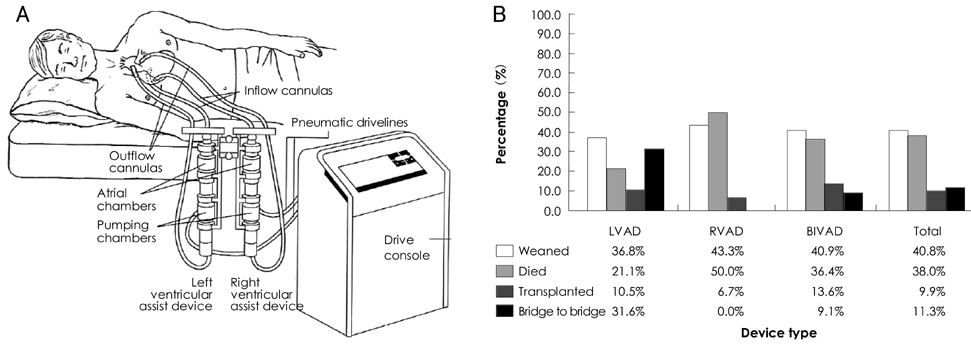
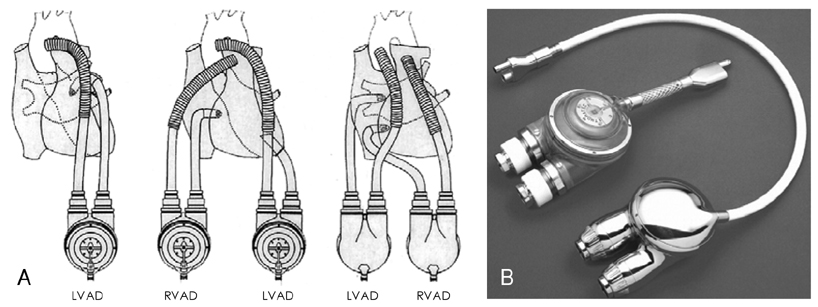
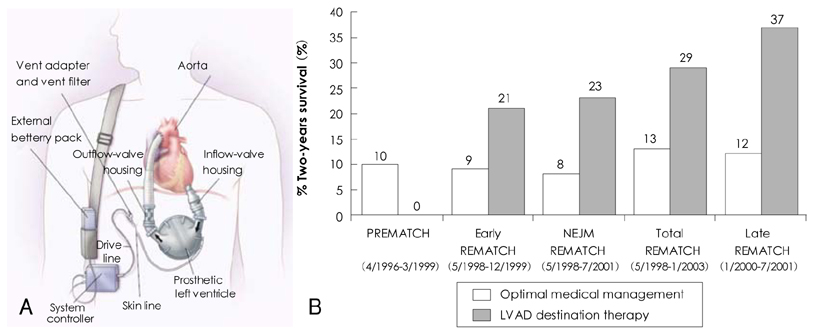
Figure
Cited by 1 articles
-
Pediatric Ventricular Assist Device
Yu Rim Shin, Young-Hwan Park, Han Ki Park
Korean Circ J. 2019;49(8):678-690. doi: 10.4070/kcj.2019.0163.
Reference
-
1. DeBakey ME. Development of mechanical heart devices. Ann Thorac Surg. 2005. 79:S2228–S2231.2. DeBakey ME. Left ventricular bypass pump for cardiac assistance: clinical experience. Am J Cardiol. 1971. 27:3–11.3. Cooley DA, Liotta D, Hallman GL, Bloodwell RD, Leachman RD, Milam JD. Orthotopic cardiac prosthesis for two staged cardiac replacement. Am J Cardiol. 1969. 24:723–730.4. Heart Disease and Stroke Statistics: 2004 update. 2004. Dallas: American Heart Association;24–25.5. Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction: etiologies, management and outcome. J Am Coll Cardiol. 2000. 36:3 Suppl A. 1063–1070.6. Wheeldon DR. Mechanical circulatory support: state of the art and future perspectives. Perfusion. 2003. 18:233–243.7. DiGiorgi P, Rao V, Naka Y, Oz M. Which patient, which pump? J Heart Lung Transplant. 2003. 22:221–235.8. Deng MC, Naka Y. Mechanical Circulatory Support Therapy in Advanced Heart Failure. 2007. London: Imperial College Press;40–69.9. Chandra D, Kar B, Idelchik G, et al. Usefulness of percutaneuous left ventricular assist device as a bridge to recovery from myocarditis. Am J Cardiol. 2007. 99:1755–1756.10. Siegenthaler MP, Brehm K, Strecker T, et al. The Impella Recover microaxial left ventricular assist device reduces mortality for postcardiotomy failure: a three-center experience. J Thorac Cardiovasc Surg. 2004. 127:812–822.11. Agati S, Ciccarello G, Ocello S, et al. Pulsatile ECMO and VAD: a dual use of a new device in pediatric cardiac patients. ASAIO J. 2006. 52:501–504.12. Morgan JA, Stewart AS, Lee BJ, Oz MC, Naka Y. Role of the Abiomed BVS 5000 device for short-term support and bridge to transplantation. ASAIO J. 2004. 50:360–363.13. Magliato K, Kleisli T, Soukiasian H, et al. Biventricular support in patients with profound cardiogenic shock: single center experience. ASAIO J. 2003. 49:475–479.14. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001. 345:1435–1443.15. Leitz K, Miller LW. Will left-ventricular assist device therapy replace heart transplantation in the foreseeable future? Curr Opin Cardiol. 2005. 20:132–137.16. Pae WE, Connell JM, Adelowo A, et al. Does total implantability reduce infection with the use of a left ventricular assist device? J Heart Lung Transplant. 2007. 26:219–229.17. Rogers JG, Butler J, Lansman SL, et al. Chronic mechanical circulatory support for inotrope-depedent heart failure patients who are not transplant candidates. J Am Coll Cardiol. 2007. 50:741–747.18. Deng MC, Edwards LB, Hertz MI, et al. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: third annual report-2005. J Heart Lung Transplant. 2005. 24:1182–1187.19. McMurray JJ, Stewart S. Epidemiology, aetiology and prognosis of heart failure. Heart. 2000. 83:596–602.20. Hosenpud JD, Bennett LE, Keck BM, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: eighteenth official report-2001. J Heart Lung Transplant. 2001. 20:805–815.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Advanced Heart Failure: Beyond Medical Treatment
- Pediatric Mechanical Circulatory Support
- Mechanical Circulatory Support for Advanced Heart Failure
- Treatment of Medically Intractable End-Stage Heart Failure
- Short-term Mechanical Circulatory Support with Centrifugal Pump in Cardiac Arrest or Cardiogenic Shock: Report of 5 cases