Yonsei Med J.
2009 Aug;50(4):517-520. 10.3349/ymj.2009.50.4.517.
Differences between the Measured and Calculated Free Serum Phenytoin Concentrations in Epileptic Patients
- Affiliations
-
- 1Department of Neurology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. kzoo@yuhs.ac
- KMID: 1758610
- DOI: http://doi.org/10.3349/ymj.2009.50.4.517
Abstract
- PURPOSE
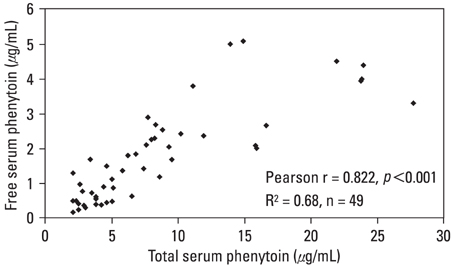
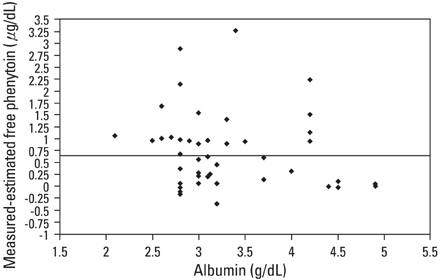
The pharmacokinetics of phenytoin is complicated by genetic and environmental differences. It is, therefore, important to monitor the serum concentrations in patients who receive phenytoin. Because most of the phenytoin in serum is bound to proteins, the level of serum albumin influences the amount of free phenytoin. MATERIALS AND METHODS: We compared the measured and calculated free phenytoin levels in epileptic patients who were taking phenytoin monotherapy, using the Sheiner-Tozer equation. A total of 49 patients (30 men and 19 women; age range, 15 - 87 years) were included in the study and their trough serum phenytoin and albumin concentrations were analyzed. RESULTS: The linear correlation between free and total phenytoin concentrations was moderate (r = 0.822, p < 0.001). The mean difference between measured and calculated free phenytoin was large (0.65 +/- 0.88 microg/mL; 95% confidence interval (CI), -1.11 to 2.41). After dividing the patients into groups by albumin concentration, hypoalbuminemic patients (< 3.5 g/dL) more often had a greater percent difference (> or = 20%) than observed in the normoalbuminemic (> or = 3.5 g/dL) group. CONCLUSION: In hypoalbuminemic patients, the measurement of free phenytoin level is necessary to properly evaluate the phenytoin level than that calculated from total phenytoin level.
Keyword
MeSH Terms
Figure
Reference
-
1. Nuwer MR, Browne TR, Dodson WE, Dreifuss FE, Engel J Jr, Leppik IE, et al. Generic substitutions for antiepileptic drugs. Neurology. 1990. 40:1647–1651.
Article2. Richens A, Dunlop A. Serum-phenytoin levels in management of epilepsy. Lancet. 1975. 2:247–248.
Article3. Kilpatrick CJ, Wanwimolruk S, Wing LM. Plasma concentrations of unbound phenytoin in the management of epilepsy. Br J Clin Pharmacol. 1984. 17:539–546.
Article4. Mackichan JJ. Pharmacokinetic consequences of drug displacement from blood and tissue proteins. Clin Pharmacokinet. 1984. 9:Suppl 1. 32–41.
Article5. Soldin SJ. Free drug measurements. When and why? An overview. Arch Pathol Lab Med. 1999. 123:822–823.6. Perucca E. Free level monitoring of antiepileptic drugs. Clinical usefulness and case studies. Clin Pharmacokinet. 1984. 9:Suppl 1. 71–78.
Article7. Bryson SM, Al-Lanqawi Y, Kelman AW, Whiting B. Comparison of a Bayesian forecasting technique with a new method for estimating phenytoin dose requirements. Ther Drug Monit. 1988. 10:80–84.
Article8. Levine M, Chang T. Therapeutic drug monitoring of phenytoin. Rationale and current status. Clin Pharmacokinet. 1990. 19:341–358.
Article9. Bailey DN, Briggs JR. The binding of selected therapeutic drugs to human serum alpha-1 acid glycoprotein and to human serum albumin in vitro. Ther Drug Monit. 2004. 26:40–43.10. Fedler C, Stewart MJ. Plasma total phenytoin: a possibly misleading test in developing countries. Ther Drug Monit. 1999. 21:155–160.11. Sheiner LB, Tozer TN. Melmon KL, Morelli HF, editors. Clinical pharmacokinetics: the use of plasma concentrations of drugs. Clinical Pharmacology: Basic Principles in Therapeutics. 1978. New York: Macmillan;71–109.12. Mlynarek ME, Peterson EL, Zarowitz BJ. Predicting unbound phenytoin concentrations in the critically ill neurosurgical patient. Ann Pharmacother. 1996. 30:219–223.
Article13. Dutkiewicz G, Wójcicki J, Gawrońska-Szklarz B. [The influence of hyperlipidemia on pharmacokinetics of free phenytoin]. Neurol Neurochir Pol. 1995. 29:203–211.14. Dasgupta A, Crossey MJ. Elevated free fatty acid concentrations in lipemic sera reduce protein binding of valproic acid significantly more than phenytoin. Am J Med Sci. 1997. 313:75–79.
Article15. Doucet J, Fresel J, Hue G, Moore N. Protein binding of digitoxin, valproate and phenytoin in sera from diabetics. Eur J Clin Pharmacol. 1993. 45:577–579.
Article16. Hooper WD, Bochner F, Eadie MJ, Tyrer JH. Plasma protein binding of diphenylhydantoin. Effects of sex hormones, renal and hepatic disease. Clin Pharmacol Ther. 1974. 15:276–282.17. Odar-Cederlöf I, Borgå O. Kinetics of diphenylhydantoin in uraemic patients: consequences of decreased plasma protein binding. Eur J Clin Pharmacol. 1974. 7:31–37.
Article18. Banh HL, Burton ME, Sperling MR. Interpatient and intrapatient variability in phenytoin protein binding. Ther Drug Monit. 2002. 24:379–385.
Article19. Peterson GM, Khoo BH, von Witt RJ. Clinical response in epilepsy in relation to total and free serum levels of phenytoin. Ther Drug Monit. 1991. 13:415–419.
Article20. Lindow J, Wijdicks EF. Phenytoin toxicity associated with hypoalbuminemia in critically ill patients. Chest. 1994. 105:602–604.
Article21. Wolf GK, McClain CD, Zurakowski D, Dodson B, McManus ML. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006. 7:434–439.22. Monaghan MS, Marx MA, Olsen KM, Turner PD, Bergman KL. Correlation and prediction of phenytoin protein binding using standard laboratory parameters in patients after renal transplantation. Ther Drug Monit. 2001. 23:263–267.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phenytoin-Dexamethasone Interaction
- Alterations in the carnitine metabolism in epileptic children treated with valproic acid
- Coefficient Variations of Serum Levels of Phenytoin, Carbamazepine, and Valproic Acid in Compliant Epileptics
- Decreased Phenytoin Absorption in Patients with Continuous Enteral Feedings
- Parkinsonism Caused by Phenytoin Intoxication-A Case Report