Yonsei Med J.
2009 Jun;50(3):309-321. 10.3349/ymj.2009.50.3.309.
Promoter Methylation in the Genesis of Gastrointestinal Cancer
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Sammons Cancer Center, Baylor Research Institute, Baylor University Medical Center, Dallas, Texas, USA. RickBo@BaylorHealth.edu
- 2Division of Gastroenterology, Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1758556
- DOI: http://doi.org/10.3349/ymj.2009.50.3.309
Abstract
- Colorectal cancers (CRC)-and probably all cancers-are caused by alterations in genes. This includes activation of oncogenes and inactivation of tumor suppressor genes (TSGs). There are many ways to achieve these alterations. Oncogenes are frequently activated by point mutation, gene amplification, or changes in the promoter (typically caused by chromosomal rearrangements). TSGs are typically inactivated by mutation, deletion, or promoter methylation, which silences gene expression. About 15% of CRC is associated with loss of the DNA mismatch repair system, and the resulting CRCs have a unique phenotype that is called microsatellite instability, or MSI. This paper reviews the types of genetic alterations that can be found in CRCs and hepatocellular carcinoma (HCC), and focuses upon the epigenetic alterations that result in promoter methylation and the CpG island methylator phenotype (CIMP). The challenge facing CRC research and clinical care at this time is to deal with the heterogeneity and complexity of these genetic and epigenetic alterations, and to use this information to direct rational prevention and treatment strategies.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Clinical Implications of Microsatellite Instability in T1 Colorectal Cancer
Jeonghyun Kang, Hak Woo Lee, Im-kyung Kim, Nam Kyu Kim, Seung-Kook Sohn, Kang Young Lee
Yonsei Med J. 2015;56(1):175-181. doi: 10.3349/ymj.2015.56.1.175.Lack of Aberrant Methylation in an Adjacent Area of Left-Sided Colorectal Cancer
Otgontuya Sambuudash, Hyun-Soo Kim, Mee Yon Cho
Yonsei Med J. 2017;58(4):749-755. doi: 10.3349/ymj.2017.58.4.749.
Reference
-
1. Vogelstein B, Fearon ER, Kern SE, Hamilton SR, Preisinger AC, Nakamura Y, et al. Allelotype of colorectal carcinomas. Science. 1989. 244:207–211.
Article2. Goel A, Arnold CN, Niedzwiecki D, Chang DK, Ricciardiello L, Carethers JM, et al. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003. 63:1608–1614.3. Li R, Sonik A, Stindl R, Rasnick D, Duesberg P. Aneuploidy vs. gene mutation hypothesis of cancer: recent study claims mutation but is found to support aneuploidy. Proc Natl Acad Sci U S A. 2000. 97:3236–3241.
Article4. Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997. 386:623–627.
Article5. Nishida N, Nagasaka T, Kashiwagi K, Boland CR, Goel A. High copy amplification of the Aurora-A gene is associated with chromosomal instability phenotype in human colorectal cancers. Cancer Biol Ther. 2007. 6:525–533.
Article6. Rodriguez J, Frigola J, Vendrell E, Risques RA, Fraga MF, Morales C, et al. Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Res. 2006. 66:8462–9468.
Article7. Sieber OM, Heinimann K, Gorman P, Lamlum H, Crabtree M, Simpson CA, et al. Analysis of chromosomal instability in human colorectal adenomas with two mutational hits at APC. Proc Natl Acad Sci U S A. 2002. 99:16910–16915.
Article8. Boland CR, Komarova NL, Goel A. Chromosomal instability and cancer: not just one CINgle mechanism. Gut. 2009. 58:163–164.
Article9. Muleris M, Chalastanis A, Meyer N, Lae M, Dutrillaux B, Sastre-Garau X, et al. Chromosomal instability in near-diploid colorectal cancer: a link between numbers and structure. PLoS ONE. 2008. 3:e1632.
Article10. Duval A, Reperant M, Compoint A, Seruca R, Ranzani GN, Iacopetta B, et al. Target gene mutation profile differs between gastrointestinal and endometrial tumors with mismatch repair deficiency. Cancer Res. 2002. 62:1609–1612.11. Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995. 268:1336–1338.
Article12. Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997. 275:967–969.
Article13. Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008. 135:1079–1099.
Article14. Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999. 96:8681–8686.
Article15. Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003. 349:2042–2054.
Article16. Yamashita K, Dai T, Dai Y, Yamamoto F, Perucho M. Genetics supersedes epigenetics in colon cancer phenotype. Cancer Cell. 2003. 4:121–131.
Article17. Ahuja N, Mohan AL, Li Q, Stolker JM, Herman JG, Hamilton SR, et al. Association between CpG island methylation and microsatellite instability in colorectal cancer. Cancer Res. 1997. 57:3370–3374.18. Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997. 57:808–811.19. Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006. 38:787–793.
Article20. Eads CA, Danenberg KD, Kawakami K, Saltz LB, Danenberg PV, Laird PW. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999. 59:2302–2306.21. Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, Murtaugh MA, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005. 129:837–845.22. Goel A, Nagasaka T, Arnold CN, Inoue T, Hamilton C, Niedzwiecki D, et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007. 132:127–138.23. Ogino S, Odze RD, Kawasaki T, Brahmandam M, Kirkner GJ, Laird PW, et al. Correlation of pathologic features with CpG island methylator phenotype (CIMP) by quantitative DNA methylation analysis in colorectal carcinoma. Am J Surg Pathol. 2006. 30:1175–1183.24. Tuupanen S, Karhu A, Järvinen H, Mecklin JP, Launonen V, Aaltonen LA. No evidence for dual role of loss of heterozygosity in hereditary non-polyposis colorectal cancer. Oncogene. 2007. 26:2513–2517.25. Kaz A, Kim YH, Dzieciatkowski S, Lynch H, Watson P, Kay Washington M, et al. Evidence for the role of aberrant DNA methylation in the pathogenesis of Lynch syndrome adenomas. Int J Cancer. 2007. 120:1922–1929.26. Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007. 318:1108–1113.27. Nagasaka T, Goel A, Notohara K, Takahata T, Sasamoto H, Uchida T, et al. Methylation pattern of the O6-methylguanine-DNA methyltransferase gene in colon during progressive colorectal tumorigenesis. Int J Cancer. 2008. 122:2429–2436.
Article28. Forrester K, Almoguera C, Han K, Grizzle WE, Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. Nature. 1987. 327:298–303.
Article29. Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987. 327:293–297.
Article30. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002. 417:949–954.
Article31. Nagasaka T, Koi M, Kloor M, Gebert J, Vilkin A, Nishida N, et al. Mutations in both KRAS and BRAF may contribute to the methylator phenotype in colon cancer. Gastroenterology. 2008. 134:1950.e1–1960.e1.
Article32. Aaltonen LA, Peltomäki P, Mecklin JP, Järvinen H, Jass JR, Green JS, et al. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994. 54:1645–1648.33. Tannergård P, Liu T, Weger A, Nordenskjöld M, Lindblom A. Tumorigenesis in colorectal tumors from patients with hereditary non-polyposis colorectal cancer. Hum Genet. 1997. 101:51–55.
Article34. Esteller M, Fraga MF, Guo M, Garcia-Foncillas J, Hedenfalk I, Godwin AK, et al. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet. 2001. 10:3001–3007.
Article35. Sanchez de Abajo A, de la Hoya M, van Puijenbroek M, Godino J, Díaz-Rubio E, Morreau H, et al. Dual role of LOH at MMR loci in hereditary non-polyposis colorectal cancer? Oncogene. 2006. 25:2124–2130.
Article36. Kim WS, Son HJ, Park JO, Song SY, Park C. Promoter methylation and down-regulation of DAPK is associated with gastric atrophy. Int J Mol Med. 2003. 12:827–830.37. Garcia-Manero G, Bueso-Ramos C, Daniel J, Williamson J, Kantarjian HM, Issa JP. DNA methylation patterns at relapse in adult acute lymphocytic leukemia. Clin Cancer Res. 2002. 8:1897–1903.38. Shen L, Ahuja N, Shen Y, Habib NA, Toyota M, Rashid A, et al. DNA methylation and environmental exposures in human hepatocellular carcinoma. J Natl Cancer Inst. 2002. 94:755–761.
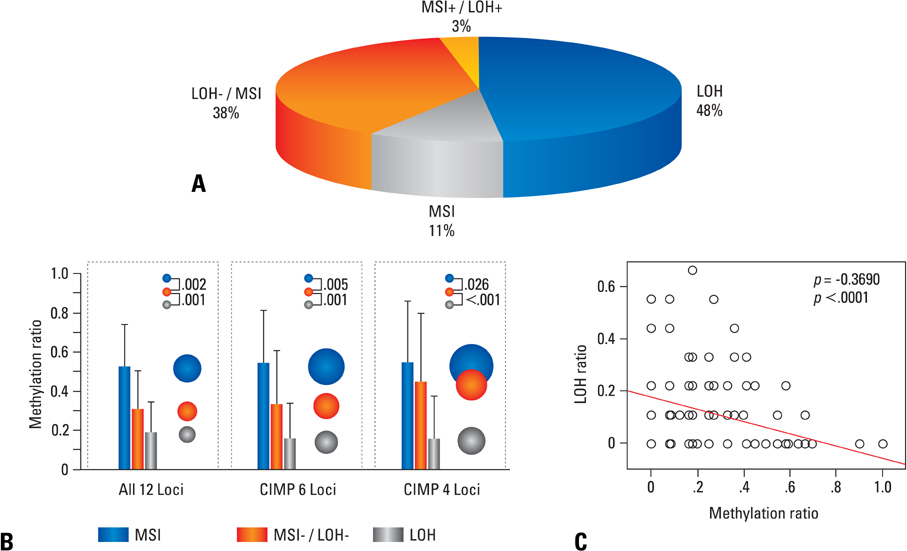
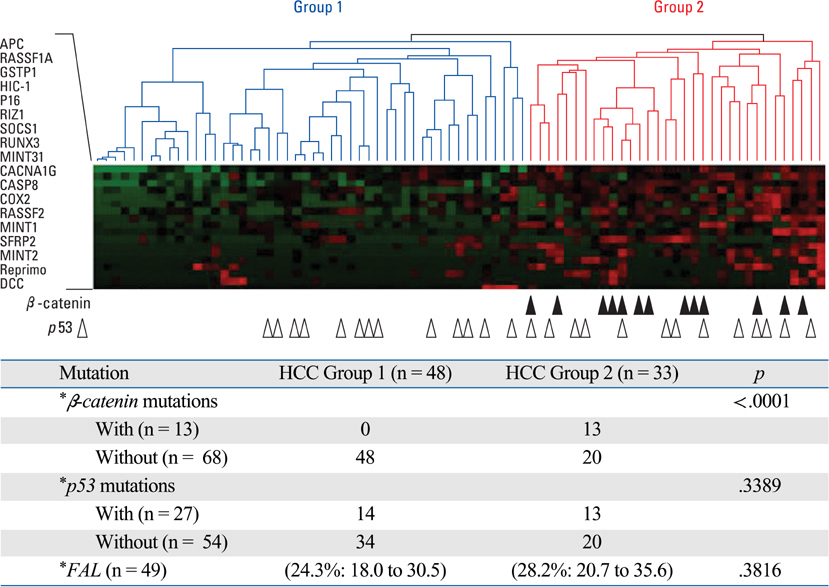
Article39. Nishida N, Nishimura T, Nagasaka T, Ikai I, Goel A, Boland CR. Extensive methylation is associated with beta-catenin mutations in hepatocellular carcinoma: evidence for two distinct pathways of human hepatocarcinogenesis. Cancer Res. 2007. 67:4586–4594.
Article40. Nishida N, Nagasaka T, Nishimura T, Ikai I, Boland CR, Goel A. Aberrant methylation of multiple tumor suppressor genes in aging liver, chronic hepatitis, and hepatocellular carcinoma. Hepatology. 2008. 47:908–918.41. Greger V, Passarge E, Höpping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989. 83:155–158.
Article42. Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000. 1:11–19.
Article43. Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002. 3:662–673.
Article44. Slattery ML, Curtin K, Sweeney C, Levin TR, Potter J, Wolff RK, et al. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2007. 120:656–663.
Article45. Samowitz WS, Albertsen H, Sweeney C, Herrick J, Caan BJ, Anderson KE, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006. 98:1731–1738.
Article46. Kaneko Y, Sakurai S, Hironaka M, Sato S, Oguni S, Sakuma Y, et al. Distinct methylated profiles in Helicobacter pylori dependent and independent gastric MALT lymphomas. Gut. 2003. 52:641–646.47. Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006. 12:989–995.
Article48. Chan AO, Peng JZ, Lam SK, Lai KC, Yuen MF, Cheung HK, et al. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006. 55:463–468.
Article49. Perri F, Cotugno R, Piepoli A, Merla A, Quitadamo M, Gentile A, et al. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. Pylori infected patients and effect of eradication. Am J Gastroenterol. 2007. 102:1361–1371.
Article50. Goel A, Li MS, Nagasaka T, Shin SK, Fuerst F, Ricciardiello L, et al. Association of JC virus T-antigen expression with the methylator phenotype in sporadic colorectal cancers. Gastroenterology. 2006. 130:1950–1961.
Article51. Herath NI, Leggett BA, MacDonald GA. Review of genetic and epigenetic alterations in hepatocarcinogenesis. J Gastroenterol Hepatol. 2006. 21:15–21.
Article52. Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, et al. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002. 160:787–794.
Article53. Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007. 39:237–242.
Article54. Ohm JE, Baylin SB. Stem cell chromatin patterns: an instructive mechanism for DNA hypermethylation? Cell Cycle. 2007. 6:1040–1043.
Article55. Rashid A, Shen L, Morris JS, Issa JP, Hamilton SR. CpG island methylation in colorectal adenomas. Am J Pathol. 2001. 159:1129–1135.
Article56. Chan AO, Broaddus RR, Houlihan PS, Issa JP, Hamilton SR, Rashid A. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol. 2002. 160:1823–1830.
Article57. Shin SK, Nagasaka T, Jung BH, Matsubara N, Kim WH, Carethers JM, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007. 133:1849–1857.
Article58. Ahlquist DA, Skoletsky JE, Boynton KA, Harrington JJ, Mahoney DW, Pierceall WE, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000. 119:1219–1227.
Article59. Müller HM, Oberwalder M, Fiegl H, Morandell M, Goebel G, Zitt M, et al. Methylation changes in faecal DNA: a marker for colorectal cancer screening? Lancet. 2004. 363:1283–1285.
Article60. Nagasaka T, Goel A, Matsubara N, Tanaka N. Detection of fecal DNA methylation for colorectal neoplasia: does it lead to an optimal screening test? Acta Med Okayama. 2006. 60:249–256.61. Chen WD, Han ZJ, Skoletsky J, Olson J, Sah J, Myeroff L, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005. 97:1124–1132.
Article62. Zou H, Taylor WR, Harrington JJ, Hussain FT, Cao X, Loprinzi CL, et al. High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology. 2009. 136:459–470.
Article63. Esteller M. Epigenetics in cancer. N Engl J Med. 2008. 358:1148–1159.
Article64. Mitsuno M, Kitajima Y, Ide T, Ohtaka K, Tanaka M, Satoh S, et al. Aberrant methylation of p16 predicts candidates for 5-fluorouracil-based adjuvant therapy in gastric cancer patients. J Gastroenterol. 2007. 42:866–873.
Article65. Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008. 123:8–13.66. Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003. 106:66–73.
Article67. Oki Y, Issa JP. Review: recent clinical trials in epigenetic therapy. Rev Recent Clin Trials. 2006. 1:169–182.
Article68. Kaminskas E, Farrell A, Abraham S, Baird A, Hsieh LS, Lee SL, et al. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res. 2005. 11:3604–3608.
Article69. Hellebrekers DM, Griffioen AW, van Engeland M. Dual targeting of epigenetic therapy in cancer. Biochim Biophys Acta. 2007. 1775:76–91.70. Walker DL, Padgett BL, ZuRhein GM, Albert AE, Marsh RF. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973. 181:674–676.71. Gordon J, Khalili K. The human polyomavirus, JCV, and neurological diseases (review). Int J Mol Med. 1998. 1:647–655.
Article72. Boland CR, Luciani MG, Gasche C, Goel A. Infection, inflammation, and gastrointestinal cancer. Gut. 2005. 54:1321–1331.
Article73. Laghi L, Randolph AE, Chauhan DP, Marra G, Major EO, Neel JV, et al. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc Natl Acad Sci U S A. 1999. 96:7484–7489.
Article74. Ricciardiello L, Laghi L, Ramamirtham P, Chang CL, Chang DK, Randolph AE, et al. JC virus DNA sequences are frequently present in the human upper and lower gastrointestinal tract. Gastroenterology. 2000. 119:1228–1235.
Article75. Jung WT, Li MS, Goel A, Boland CR. JC virus T-antigen expression in sporadic adenomatous polyps of the colon. Cancer. 2008. 112:1028–1036.
Article76. Enam S, Del Valle L, Lara C, Gan DD, Ortiz-Hidalgo C, Palazzo JP, et al. Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002. 62:7093–7101.77. Casini B, Borgese L, Del Nonno F, Galati G, Izzo L, Caputo M, et al. Presence and incidence of DNA sequences of human polyomaviruses BKV and JCV in colorectal tumor tissues. Anticancer Res. 2005. 25:1079–1085.78. Theodoropoulos G, Panoussopoulos D, Papaconstantinou I, Gazouli M, Perdiki M, Bramis J, et al. Assessment of JC polyoma virus in colon neoplasms. Dis Colon Rectum. 2005. 48:86–91.
Article79. Ricciardiello L, Baglioni M, Giovannini C, Pariali M, Cenacchi G, Ripalti A, et al. Induction of chromosomal instability in colonic cells by the human polyomavirus JC virus. Cancer Res. 2003. 63:7256–7262.80. Goel A, Nagasaka T, Arnold CN, Inoue T, Hamilton C, Niedzwiecki D, et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007. 132:127–138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RUNX3 Methylation Status in Colonic Carcinoma and Adenoma
- AKAP12alpha is Associated with Promoter Methylation in Lung Cancer
- Promoter Methylation of p16 Gene in Cervical Cancer
- Analysis of Epigenetic Marker of Bladder Cancer
- Peripheral blood BRCA1 methylation profiling to predict familial ovarian cancer