Cancer Res Treat.
2005 Feb;37(1):37-43.
A Preliminary Results of a Randomized Trial Comparing Monthly 5-flourouracil and Cisplatin to Weekly Cisplatin Alone Combined with Concurrent Radiotherapy for Locally Advanced Cervical Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea. ekchoi@amc.seoul.kr
- 2Department of Obstetrics and Gynecology, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea.
- 3Department of Statistics, Inha University, Incheon, Korea.
Abstract
- PURPOSE
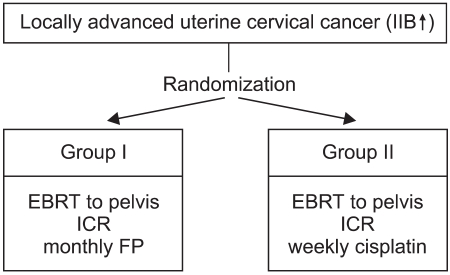
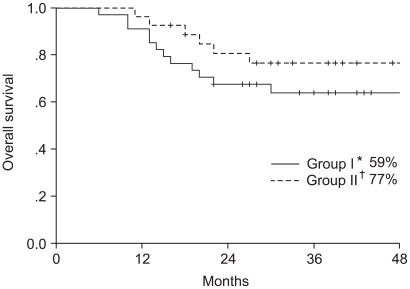
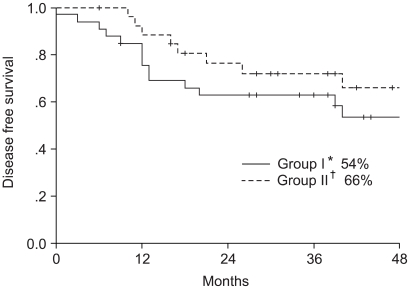
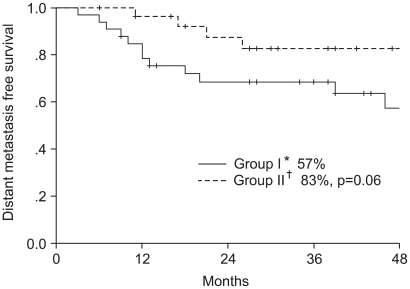
To determine the superior chemotherapeutic regimen between monthly 5-FU plus cisplatin (FP) and weekly cisplatin alone in concurrent chemoradiotherapy for locally advanced cervical cancer, the compliance of treatment, response, survival and toxicities were analyzed between the two arms. MATERIALS AND METHODS: Between March 1998 and December 2001, 61 patients with locally advanced cervical cancer (stage IIB through IVA) and negative para-aortic lymph nodes were randomly assigned to either `monthly FP' (arm I, n=34) or `weekly cisplatin' (arm II, n=27) with concurrent radiotherapy. The patients of arm I received FP (5-FU 1, 000 mg/m2/day+cisplatin 20 mg/m2/day, for 5 days, for 3 cycles at 4 week intervals) and those of arm II received cisplatin (30 mg/m2/day, for 6 cycles at 1 week intervals) with concurrent radiotherapy. The radiotherapy consisted of 41.4~50.4 Gy external beam irradiation in 23~28 fractions to the whole pelvis, with high dose rate brachytherapy delivering a dose of 30~35 Gy in 6~7 fractions to point A. During the brachytherapy, a parametrial boost was delivered. The median follow-up period for survivors was 44 months. RESULTS: The compliance of treatment in monthly FP weekly cisplatin arms were 62 and 81%, respectively. The complete response rates at 3 months were 96 and 88% in arms I and II, respectively. The 4-year overall survival and disease free survival rates were 64 and 54% in the arm I and 77 and 66% in the arm II, respectively. The incidence of hematologic toxicity more than grade 2 was 29% in the arm I and 15% in the arm II. Only one patient in arm I experienced grade 3 gastrointestinal toxicity. No severe genitourinary toxicity was observed. CONCLUSION: No significant difference was observed in the compliance, responses, survival rates and acute toxicities between the two treatment arms. More patients and further follow up will be required.
MeSH Terms
Figure
Reference
-
1. Gonzalez DG, Ketting BW, Bunningen BV, Dijk JD. Carcinoma of the uterine cervix stage IB and IIA: result of postoperative irradiation in patients with microscopic infiltration in the parametrium and/or lymph node metastasis. Int J Radiat Oncol Biol Phys. 1989; 16:389–395. PMID: 2921143.2. Coia L, Won M, Lanciano R, Marcial VA, Martz K, Hanks G. The patterns of care outcome study for cancer of the uterine cancer: Results of the second national practice survey. Cancer. 1990; 66:2451–2456. PMID: 2249184.3. Jampolis S, Andras EJ, Fletcher GH. Analysis of sites and causes of failures of irradiation in invasive squamous cell carcinoma of the intact uterine cervix. Radiology. 1975; 115:681–685. PMID: 1129485.
Article4. Leibel S, Bauer M, Wasserman T, Marcial V, Rotman M, Hornback N, et al. Radiotherapy with or without misonidazole in patients with stage IIIB or IVA squamous cell carcinoma of the uterine cervix: preliminary report of a radiation therapy oncology group randomized trial. Int J Radiat Oncol Biol Phys. 1987; 13:541–549. PMID: 3104249.5. Piver MS, Barlow JJ, Vongtama V, Blumenson L. Hydroxyurea: a radiation potentiator in carcinoma of the uterine cervix. A randomized double-blind study. Am J Obstet Gynecol. 1983; 147:803–808. PMID: 6359885.6. Dewit L. Combined treatment of radiation and cisdiaminedichloroplatinum (II): a review of experimental and clinical data. Int J Radiat Oncol Biol Phys. 1987; 13:403–426. PMID: 3549645.7. Tattersall MH, Lorvidhaya V, Vootiprux V, Cheirsilpa A, Wong F, Azhar T, et al. Randomized trial of epirubicin and cisplatin chemotherapy followed by pelvic radiation in locally advanced cervical cancer. J Clin Oncol. 1995; 13:444–451. PMID: 7844607.8. Kumar L, Kaushal R, Nandy M, Biswal BM, Kumar S, Kriplani A, et al. Chemotherapy followed by radiotherapy versus radiotherapy alone in locally advanced cervical cancer: a randomized study. Gynecol Oncol. 1994; 54:307–315. PMID: 7522200.
Article9. Souhami L, Gil RA, Allan SE, Canary PC, Araujo CM, Pinto LH, et al. A randomized trial of chemotherapy followed by pelvic radiation therapy in stage IIIB carcinoma of the cervix. J Clin Oncol. 1991; 9:970–977. PMID: 1709686.
Article10. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999; 340:1137–1143. PMID: 10202164.
Article11. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999; 340:1144–1153. PMID: 10202165.
Article12. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjuvant to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999; 17:1339–1348. PMID: 10334517.13. National Cancer Institute. Concurrent chemoradiation for cervical cancer. Clinical announcement. 1999. 2.14. Perez CZ, Grigsby PW, Chao CKS. Chemotherapy and irradiation in locally advanced squamous cell carcinoma of the uterine cervix: A review. Semin Radiat Oncol. 1997; 7(Supple 2):45–65.
Article15. Fu KK. Biological basis for the interaction of chemotherapeutic agents and radiation therapy. Cancer. 1985; 55(9 Suppl):2123–2130. PMID: 3884135.
Article16. Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet. 2000; 355:949–955. PMID: 10768432.
Article17. Eapen L, Stewart D, Danjoux C, Genest P, Futter N, Moors D, et al. Intraarterial cisplatin and concurrent radiation for locally advanced bladder cancer. J Clin Oncol. 1989; 7:230–235. PMID: 2915239.
Article18. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999; 340:1154–1161. PMID: 10202166.
Article19. Peters WA, Liu PY, Barrett RJ, Barrett R, Gordon W, Stock R, et al. Cisplatin and 5-fluorouracil plus radiation therapy are superior to radiation therapy as adjunctive in high-risk early-stage carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: Report of a phase III intergroup study. Gynecol Oncol. 1999; 72:443–527. PMID: 10053123.20. Fyles A, Keane TJ, Barton M, Simm J. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol. 1992; 25:273–279. PMID: 1480773.
Article21. Lanciano RM, Pajak TF, Martz K, Hanks GE. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a patterns-of-care study. Int J Radiat Oncol Biol Phys. 1993; 25:391–397. PMID: 8436516.
Article22. Petereit DG, Sarkaria JN, Chappell R, Fowler JF, Hartmann TJ, Kinsella TJ, et al. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1995; 32:1301–1307. PMID: 7635769.
Article23. Choy D, Wong LC, Sham J, Ngan HY, Ma HK. Dose-tumor response of carcinoma of cervix: an analysis of 594 patients treated by radiotherapy. Gynecol Oncol. 1993; 49:311–317. PMID: 8314532.24. Jurado M, Monge RM, Foncillas JG, Azinovic I, Aristu J, Garcia GL, et al. Pilot study of concurrent cisplatin, 5-fluorouracil, and external beam radiotherapy prior to radical surgery +/- intraoperative electron beam radiotherapy in locally advanced cervical cancer. Gynecol Oncol. 1999; 74:30–37. PMID: 10385548.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of concurrent chemoradiation therapy with weekly cisplatin versus monthly fluorouracil plus cisplatin in FIGO stage IIB-IVA cervical cancer
- Concurrent Chemoradiotherapy in Locally Advanced Carcinoma of the Uterine Cervix Preliminary Results of Phases III Prospective Randomized Trial
- Comparison of concurrent chemoradiotherapy with cisplatin plus 5-fluorouracil versus cisplatin plus paclitaxel in patients with locally advanced cervical carcinoma
- Efficacy and safety of cisplatin combined with paclitaxel concurrent radiotherapy in patients with locally advanced cervical squamous cell carcinoma
- Weekly cisplatin or gemcitabine concomitant with radiation in the management of locally advanced carcinoma cervix: results from an observational study